
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
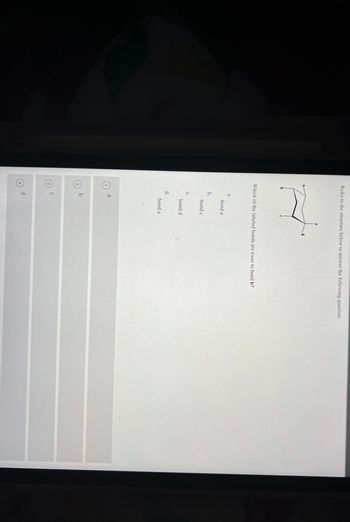
Transcribed Image Text:Refer to the structure below to answer the following question
H
Which of the labeled bonds are trans to bond b?
2
3
a.
b.
C.
d.
a
b
C
d
bond a
bond c
bond d
d
bond e
Expert Solution
arrow_forward
Step 1
-> (1,4),(1,2)=> (a,a) ,(e,e) -> trans
-> (1,4),(1,2) => (a,e),(e,a) -> cis
-> (1,3)=> (a,a),(e,e) -> cis
-> (1,3)=> (a,e),(e,a) -> trans
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Predict the shape (molecular geometry) of the following molecules or ions by filling up the table below: No. of No. of bonding pairs Molecule/ Lewis structure No. of Electron pair geometry Molecular Valence electrons Ion non- geometry bonding pairs 1. H2Searrow_forwardWhat’s the most pillar bond in this?arrow_forwardBond Energies depend on structures of covalent compounds. A positive value of a bond energy means: Select one: O a. Energy input is required to break a covalent bond b. Energy output results from forming a covalent bond c. Bond energies provide an estimate of bond strength d. All of the above are correctarrow_forward
- Which one of the following statements is false? a. ionic bonding results from the trnasfer of electrons from one atoms to another b. dipole moments result from the unequal distribution of electrons in a molecule c. the electrons in a polar bond are found nearer to the more electronegative element d. a molecule with very polar bonds can be nonpolar e. linear molecules cannot have a net dipole momentarrow_forwardDraw the molecule NO2-. What is the bonding environment around the central atom N, the number and type of bonding and nonbonding pairs A.4 single bonds B.3 single bonds C.2 double bonds D.1 double bond, a single bond and a nonbonding pairarrow_forwardWhich statement A-D about VSEPR theory is not correct? Select one: a. The steric number has five values from 2 to 6. b. Statements A-D are all correct. c. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion. d. The steric number of a central atom is the sum of the number of bonds and lone pairs around the atom. e. The molecular shape or geometry can differ from the electron-pair geometry.arrow_forward
- Which of the following bonds is least polar? Use the periodic table only. B-C b. C-F C-O a. C.arrow_forwardWhich statement A-D about VSEPR theory is not correct? Select one: a. The molecular shape or geometry can differ from the electron-pair geometry. b. In VSEPR theory, the shape or geometry of a molecule is determined by electron-electron repulsion. c. The steric number of a central atom is the sum of the number of bonded atoms and lone pairs around the central atom. d. The steric number has five values from 2 to 6. e. Statements A-D are all correct. Clear my choicearrow_forwardI would like help with this question please!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY