
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:learning|catalytics
TM
Caitlyn Kelenske | Log out
Session 53588886
Jump to ▼
3
5
7
9.
10
multiple choice question
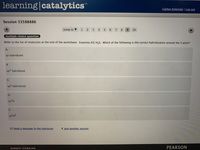
Refer to the list of molecules at the end of the worksheet. Examine #2) H2S. Which of the following is the correct hybridization around the S atom?
A.
sp hybridized
В.
sp2 hybridized
С.
sp3 hybridized
D.
sp³d
E.
sp³d2
9 Send a message to the instructor
< Join another session
ALWAYS LEARNING
PEARSON
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- complete everything 1. NO3- magic number on central atom electronic geometry molecular geometry bond angle on central agom Hybridization of central atomarrow_forward20. Which of the following statements about hybridization is FALSE? A) The ideal angle between sp³-hybridized orbitals is 109.5º. A 3s orbital is spherical with no nodes. B D) E) Carbon is not the only atom that undergoes hybridization. Mixing three atomic orbitals always results in the formation of three orbitals. In carbon, electrons in an sp²-hybridized orbital are higher in ener an sp-hybridized orbital. 21. Which of the following statements about Aspartame (sh НО. NH₂ re 6 n-bonds. 2-hybridized c bonds Oarrow_forward5. Among the most common over-the-counter drugs you might find in a medicine cabinet are mild pain relievers such ibuprofen (Advil, Motrin), naproxen (Aleve), and. acetaminophen (Tylenol). HO HO. HO. CH3 Ibuprofen Naproxen Acetaminophen a. How many sp3 hybridized carbons does each molecule have? b. How many sp2hybridized carbons does eacch molecule have? C. Can you spot any similarities in their structures?arrow_forward
- Specify the hybridization at the atoms labelled a-d. For each atom enter one of the following: sp3, dsp2, sp2, sp3d, or sp. Hybridizations are normally written with superscripted numbers, but OWL expects non-superscripted answers to this question. H H. °C H. H C N- c_b d a a) c) d)arrow_forwardSpecies # of Valence Atomic orbital of Hybridized orbitals formed Atomic orbitals Hybridization electron electron valence electrons unhybridized domains configuration CIF41- NO31- NO21- 2. SeCls1-arrow_forwardH H 8. Consider the molecule below. Determine the hybridization at each of the 2 labeled carbons. (2 CH3 a. C1 = sp3, b. C1 = sp2, C2 = sp2 C2 = sp² C2 = sp3 C2 = sp³d e. C1 = sp2, C2 = sp³d c. C1 = sp2, d. C1 = sp3d,arrow_forward
- 2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forwardWhat is the total number of carbon atoms in this molecule? What is the total number of Hydrogen atoms in this molecule? i. How many sp3, sp2,and sp hybridized carbon atoms are in this compound?arrow_forward23.) Match the molecules or ions listed with the correct hybridization about the central atom. The numbers for the hybridizations are all superscripts. For example sp2 is sp2 and sp3 is sp3. Group of answer choices carbon disulfide CS2 (2 is subscript) bromine trifluoride sulfur dioxide CHCl3 (3 is subscript)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY