
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
In all three open spots, provide the structure of the β-hydroxy ketone and α,β-unsaturated ketone that will be formed during this Crossed Aldol Condensation. (2 structures in each open spot)
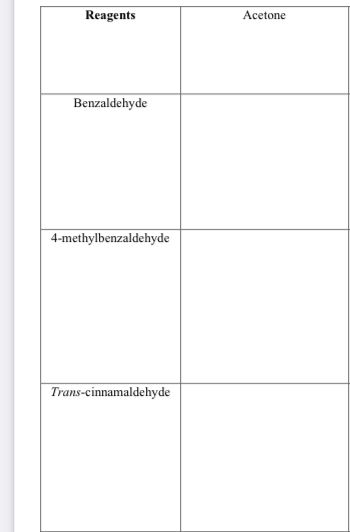
Transcribed Image Text:Reagents
Benzaldehyde
4-methylbenzaldehyde
Trans-cinnamaldehyde
Acetone
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- draw the entire synthesis of ( 8 - Hydroxy-2-isopropyl-3,4-dihydroisoquinolin-1 (2H)-one) as mentioned in the picture ( with arrows, lone pairs, substitution and elimination) 1. Start with benzaldehyde: Benzaldehyde + Ethyl acetoacetate → Chalcone This reaction is a Claisen-Schmidt condensation. The Chalcone obtained has a benzene ring and a three-carbon side chain. 2. Next, perform a Pictet-Spengler reaction: Chalcone + Ethylamine → Isoquinoline derivative This reaction forms the isoquinoline ring system. 3. Hydrogenate the isoquinoline derivative: Isoquinoline derivative + H2/Pd-C 3,4-dihydroisoquinoline This step reduces the double bond in the isoquinoline ring. 4. Oxidize the 3,4-dihydroisoquinoline to obtain the desired product: 3,4-dihydroisoquinoline + KMnO4/H2O8-Hydroxy-2- isopropyl-3,4-dihydroisoquinolin-1(2H)-onearrow_forwardPlease don't provide handwriting solutionarrow_forwardPlease can you write chemical equation of aldol condensation between 3 nitrobenzaldehyde + acetophenone and after that can you write the mechansim for the reaction with arrows.arrow_forward
- Draw the major product of the aldol addition reaction between two equivalents of this ketone with the conditions provided. Ignore inorganic byproducts. 1. H3O+ 2. Neutralizing work-up &arrow_forward1) Draw all the aldol addition stereoisomers of this aldehyde ? لمه Harrow_forwardB. Dieckmann Condensations Just as there can be intramolecular Aldol condensations, so can there be intramolecular Claisen condensations. These are called Dieckmann condensations. 1) Identify all the a hydrogens in the diester shown. Draw the enolate anion(s) that form when the hydrogen(s) is/are removed. H3CH₂CO OCH₂CH3 2) Draw a mechanism and provide the product(s) formed when the anion(s) attack(s) the other carbonyl carbon in the molecule. All electron movement must be shown with arrows.arrow_forward
- See image bearrow_forwardDraw structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol or enone below. & • You do not have to consider stereochemistry. ● Draw the enolate ion in its carbanion form. ● Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple reactants using the + sign from the drop-down menu. / MILL ? ChemDoodle Ⓡ Yarrow_forwardIf butanol underwent a self aldol addition reaction, what would be the major product 2-ethyl-2-hydroxyhexanol 2-ethyl-3-hydroxyhexanol (E)-2-ethyl-2-hexenal (Z)-2-ethyl-2-hexenal Both (Z) and (E)-2-ethyl-2-hexenalarrow_forward
- Please don't provide handwriting solutionarrow_forwardPlease don't provide handwritten solutionarrow_forwardIf a student is starting with diethylmalonate then the base they would use to generate the enolate would be NaOMe NaOEt NaOH None of the above. The synthesis of dibenzalacetone is an example of Dieckmann condensation Aldol condensation Michael reaction None of the above. Starting with a 1,6 - diester and treating it with NaOEt would be an example of Aldol Condensation Claisen Condensation Dieckmann Condensation None of the above. A nucleophile that adds to the beta carbon of an alpha, beta unsaturated aldehyde or ketone would be considered Hard Soft Acidic None of the above.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY