
Concept explainers
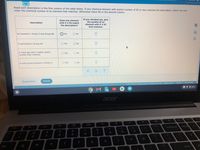
Atomic Number (Z) 92 used for Uranium. For the above mentioned condition in question we have choose elements with equal to or less than atomic number of 92.
(1) An element in period second and group 8A -
In period second Li, Be, B, C, N, O, F, Ne are present. In these elements 8A group element is Ne, Neon. It has atomic number of 10.
(2) A semimetal in group 4A -
In group 4A , the semimetal is Si, silicone. 4A group contains Carbon, silicone, germanium,tin ,lead etc in which silicone is semimetal. Silicone has atomic number of 14.
(3) A Noble gas with a higher atomic number than Chlorine -
Chlorine has atomic number of 17. Present in halogen group and period third. Argon ,At, with, atomic number 18 is higher Noble gas element than Chlorine.
(4) A main group element in period 5 -
s and p group elements are main group elements. In period 5, Rb, Sr, In, Sn, Sb, Te, I, and Xe are present.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

- Phosphorus-32 is often used to image biological tissue. Mark any/all that apply to phosphorus-32. It has 17 protons. O It symbol is 15p It has 15 electrons. It has 17 neutrons. It symbol is 32P. It has 32 protons. It has 15 protons.arrow_forwardwhy is this the correct answer when potassium has 15 protons not 19?arrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 6 and Group 7A. A metalloid in Group 5A. A noble gas with a lower atomic number than indium. A transition element in Period 2. Does any element with Z92 match the description? Yes Yes Yes O Yes O No O No O No O No If you checked yes, give the symbol of an element with Z≤ 92 that matches. X 0 Śarrow_forward
- Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description Does any element with Z ≤ 92 match the description? If you checked yes, give the symbol of an element with Z ≤ 92that matches. An element in Period 2 and Group 4A. Yes No A metalloid in Group 4A. Yes No A halogen with a higher atomic number than nitrogen. Yes No A main-group element in Period 4. Yes Noarrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 5 and Group 2A. A semimetal in Group 8A. A halogen with a higher atomic number than carbon. A main-group element in Period 4. Does any element with Z ≤ 92 match the description? Yes Yes Yes Yes No No No No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. 0 0 □ ☐arrow_forward9arrow_forward
- A robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following data on the isotopes of tin in these samples: mass isotope relative (amu) abundance 124 Sn 123.91 92.2% 119.90 7.8% 120 Sn Use these measurements to complete the entry for tin in the Periodic Table that would be used on 98765 ALEKS. Round your entry for the atomic mass to 3 significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for tin in the Periodic Table we use here on Earth. П Sn ☐arrow_forwardPlease don't provide handwritten solution ...arrow_forwardWhich of the following is true about the atom represented by the symbol: 127 53I? This atom has 53 protons, 127 neutrons and 53 electrons. 127 protons, 53 neutrons and 53 electrons. 74 protons, 53 neutrons and 127 electrons. 53 protons, 74 neutrons and 53 electrons.arrow_forward
- Read each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 6 and Group 2A. A nonmetal in Group 7A. A noble gas with a lower atomic number than lithium. A main-group element in Period 6. Does any element with Z ≤ 92 match the description? Yes OYes Yes O Yes O No O No O No O No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. X 0 3arrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes and enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 4 and Group 8A. A metalloid Group 4A. An alkaline earth metal with a lower atomic number than xenon. A main-group element in Period 3. Does any element with Z≤ 92 match the description? Yes Yes Yes Yes O O O No No No No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. 0 Si П Naarrow_forwardRead each description in the first column of the table below. If any chemical element with atomic number of 92 or less matches the description, check Yes a enter the chemical symbol of an element that matches. Otherwise check No in the second column. description An element in Period 3 and Group 2A. A nonmetal in Group 7A. An alkaline earth metal with a lower atomic number than arsenic. A main-group element in Period 6. Does any element with Z ≤ 92 match the description? Yes O Yes O Yes O Yes O No O No O No O No If you checked yes, give the symbol of an element with Z ≤ 92 that matches. X 0 0 Śarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





