
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
givin the answer for 1 & 2 below,
provide an answer for 3:
Write the balanced equation for this experiment by adding these two equations. (Note that Br2 is generated in the redox equation and consumed in the final step, thus it is considered to be a reaction intermediate and should not appear in the net equation – you will need to scale one or more of the equations before the equations are combined.)

Transcribed Image Text:1. The formation of molecular bromine (Br2) from HBr and KBrO3 must be derived using redox
reactions.
a. Write the balanced (ionic) redox equation for the conversion of BrO3 into Br2
under acidic conditions. Label the half reaction as either oxidation or reduction.
10e + 2Br03 + 12H* → Br₂ + 6H₂O (Reduction)
b. Write the balanced (ionic) redox equation for the conversion of Br into Br2 under
acidic conditions. Label the half reaction as either oxidation or reduction.
2Br → Br₂ + 2e¯ (Oxidation)
C.
Write the balanced net (ionic) redox reaction (reduced to the lowest whole
number coefficients)
6H + BrO3 + 5Br¯ → 3Br2 + 3H20
d. Rewrite the balanced net redox equation in complete (molecular) form using
KBrO3, HBr and CH3COOH as the reactants. (note that this step may require a bit
of manipulation as there are two sources of H+ HBr and CH3COOH)
KBгO3 + CH3COOH + 5HBr → 3Br2 + 3H2O + CH3COOK
2. Write the equation for the reaction of acetanilide with molecular bromine (for the organic
compounds, draw the full structures, not condensed formulae).
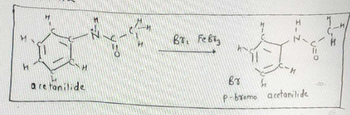
Transcribed Image Text:уго
-Z
are tanilide
Br. Febrz
H
Во
4
P-bromo acetanilide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction shown, find the limiting reactant for each of the initial quantities of reactants. express all answers as chemical forumals 2Li(s)+Br2(l)→2LiBr(s) -1 molLi ; 1 molBr2 -1.8 molLi ; 1 molBr2 -2.2 molLi ; 1 molBr2 -arrow_forwardAnswer question number four, please. Also, I would like the work wrote out if you can. Thanks so much I truly appreciate it.arrow_forwardPlease helparrow_forward
- Enter a molecular equation for the reaction of dilute sulfuric acid with iron. (Assume that sulfuric acid acts as a diprotic acid and that an iron(II) compound is formed.) Express your answer as a balanced chemical equation. Identify all of the phases in your answer. Enter a molecular equation for the reaction of hydrobromic acid with magnesium. Express your answer as a balanced chemical equation. Identify all of the phases in your answer. Enter a molecular equation for the reaction of acetic acid, CH3COOHCH3COOH, with zinc. Express your answer as a balanced chemical equation. Identify all of the phases in your answer.arrow_forwardMISSED THIS? Watch KCV: Reactions in Solutions; Read Section 5.8. You can click on the Review link to access the section in your e Text. Complete and balance each of the following equations for gas-evolution reactions. Part A HNO3(aq) + Na₂SO3(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. = | ΑΣΦ A chemical reaction does not occur for this question. Submit Part B HCl(aq) + KHCO3(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. D= | ΑΣΦ Request Answer Submit Part C A chemical reaction does not occur for this question. Request Answer ? ="= | ΑΣΦ Part D HC₂H3O2 (aq) + NaHSO3(aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. ? A chemical reaction does not occur for this question. Submit Request Answer ΑΣΦ ? (NH4)2SO4 (aq) + Sr(OH)2 (aq) → Express your answer as a chemical equation. Identify all of the phases in your answer. Submit Request Answer ? A chemical…arrow_forwardDraw all of the particles that would result if one unit of the flerovium compound, Fl(NO3)2 was completely soluble and dissociated into its ions in water.arrow_forward
- PLEASE HELParrow_forwardCan you help me with Part E-4, and Part F questions, please?arrow_forwardCheck out the following video and write the balanced equation in this question. Hint: Remember to first determine the reaction type and then write products. There are two products to this reaction, and one is an ionic compound with potassium and chlorine and one is gaseous oxygen. Please make sure to use phases for each substance and parentheses (when needed) and subscripts. https://www.youtube.com/watch?v=fLic3zmH94s&feature=emb_title (1 min 11 secs video)arrow_forward
- Directions: Write the balanced equation for each of the following situations. SHOW ALL OF YOUR WORK ON ATTACHED PAGES, OR IT WILL NOT BE ACCEPTED. In addition, list the reaction type.YOU MUST TELL THE AMOUNTS OF EVERY SUBSTANCE THAT REMAINS IN THE CONTAINER AT THE END OF THE REACTION. ASSUME THAT ALL REACTIONS GO TO COMPLETION.If only STOICHIOMETRY, tell how much of the excess reactant is used!!!! Reaction Typea. Combination Reactionb. Decomposition Reactionc. Single Displacement / THIS IS ONE TYPE OF Oxidation Reduction Reaction d. Precipitation Reactione. Gaseous Reactionf. Neutralization Reactiong. Combustion Reaction 5.92 g of sodium oxalate is reacted with 5.92 of calcium chloride follow the format used in the image provided.arrow_forwardCompare the predictions you made in the preparation activity with your observations of the actual reactions. In which reactions were you able to correctly predict the products? In which reactions did you make incorrect predictions? Were there particular reaction patterns that you had difficulty predicting correctly? Why do you think that was the case?arrow_forwardA sample of sodium carbonate solution is added to a solution of lead 2 chlorate causeing a white solid to appear. Please write the balanced molecular equation. What is the sum of the coefficients in this reaction. Make sure to add coefficients of both reactants adn both productsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY