
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
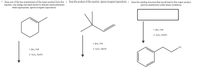
Transcribed Image Text:1.
2. Draw the product of this reaction. Ignore inorganic byproducts. 3. Draw the starting structure that would lead to this major product
Draw one of the two enantiomers of the major product from this
reaction. Use wedge and dash bonds to indicate stereochemistry
where appropriate. Ignore inorganic byproducts.
(and its enantiomer) under these conditions.
1. ВНз-ТHF
2. H2O2, NaOH
1. ВНз-ТHF
OH
1. ВНз-THF
2. H202, NaOH
2. H202, NaOH
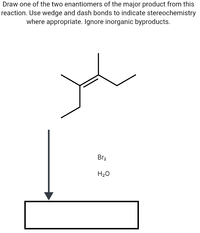
Transcribed Image Text:Draw one of the two enantiomers of the major product from this
reaction. Use wedge and dash bonds to indicate stereochemistry
where appropriate. Ignore inorganic byproducts.
Br2
H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. An epimerization is a reaction that changes the relative stereochemistry of one stereocenter. In the epimerization drawn below is have a higher or lower heat of combustion than Androstenediol? Explain using pictures. H3C OH H3C OH H3C H суу Н Androstanediol НО" НО H3C Нarrow_forwardDraw the structural formula of the product of the reaction shown below. H3C. CH3CH₂ + CH3CH₂OH **VIL • You do not have to consider stereochemistry. Na OCH₂CH3 [ ] در ? O ChemDoodle Referarrow_forwardConsider the reaction of 1,2-epoxy-1-methylcyclopentane below. It can be reacted with NaOCH3 to form product 1 or with CH3OH/H* to form product 2. 1 OCH3 a OH NaOCH3 CH₂OH CH3 give OCH 3 CH3 OH b J...CH3 OH с CH3OH CH3OH₂* CH3 OCH 3 OH d 2 OCH 3 CH3 Based on the stereochemistry shown here, product 1 would be structure [Select] and product 2 would be structure [Select]arrow_forward
- Draw the two major products of this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers. Ignore inorganic byproducts. 1. BHS-THF 2.H2O2, NaOH, H₂O 回arrow_forwardI. Fill in the Missing reagents. In some cases, more than one reaction may be required to complete a step. لی ماسه ۵۰۵۰ ملی ملی محسن Br -CH=CH₂ -C=CH ہلی - سلسلة شعبة عليه `H HC=C- Harrow_forwardOrganic Chemistry 1. Please draw out the three structures ? Please explain why one and two have meso stereoisomers, and III does not ? Thank youarrow_forward
- Match the reaction to the product. (Assume enantiomers are formed as expected.)arrow_forward2.) Which is a pair of enantiomers? CH3 H3C CI CH;CH2CH3 CH3 CH3 CH;CH;CH3 H3C CI CH;CH2CH; II II CH,CI CH;CH2CH3 H3C CI CH;CH2CH3 IV V A. I& II and III & IV В. I & II С. II & IV D. IV & Varrow_forwardDraw one of the two enantiomers of the major product from this reaction. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers. Ignore inorganic byproducts. 1. BH3-THF 2. H2O2, NaOH Qarrow_forward
- Provide the structure of the product(s) with stereochemisty if appropriate. If multiple products indicate major product or if products are formed in equal amounts. If there is no reaction expected explain why. H3C, CH3 D30* ČH3 D. 1. LDA 2. NCS H3C CH3 CH3 1. Mel CH3 H 2. D30*arrow_forwardsee these instructions. Consider stereochemistry and enter either enantiomer (but not both) if a racemic mixture of products forms. If a mixture of two diastereomers is expected, enter either (but not both). 1. MgCl 2. H₂Oarrow_forwardDraw the products of this reaction. Use a dash or wedge bond to indicate the stereochemistry substituents on asymmetric centers. Ignore inorganic byproducts. 1. Os04 NMO Select to Draw + Qarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY