
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
You are trying to determine the KM for an enzyme. Due to a lab mishap, you have only two usable data points: Use these data to calculate an approximate value for KM. Is this value likely to be an overestimate or an underestimate of the true value? Explain.
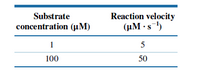
Transcribed Image Text:Reaction velocity
(µM · s-1)
Substrate
concentration (µM)
1
5
100
50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
How did you get 0.32?
Also where did you get 500 for Vmax? In the question it says 50
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
How did you get 0.32?
Also where did you get 500 for Vmax? In the question it says 50
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student was studying enzymes and wanted to track how components can impact the enzymatic activity of amylase on starch. She followed Table 10.1, listed in Lab 10 on page 104. Which of the following statements is true regarding the results of this test? The student has been instructed that something has contributed to the amylase becoming denatured, please indicate the components that could cause the impact the amylase. Question 17 options: The amylase in well B is efficiently working. The amylase in well C is efficiently working. The amylase in select wells has been denatured. The amylase in well A is efficiently working. Wells A, B, and C have enzymes that are denatured. water AgNO3 isopropyl alcoholarrow_forwardCarbon dioxide concentration (parts per million) Examine the graph and answer the following questions. Part A Atmospheric Carbon Dioxide What was the average rate of increase in carbon doxide concentration between 1900 and 19407 410 400 Express you answer in parts per million per year to two significant figures. 300 380 a ? 370 360 ppm/year 350 340 Submit Bequest Anawer 330 320 310 Part 8 Complete previous parts) 300 290 - 1860 1880 1900 1920 1940 1960 1980 2000 2020 Provide Feedback Next> Yeararrow_forwardThere are roughly 17 nutrients required by plants. (14 mineral nutrients, 3 come from air and water) List the Primary or Macro nutrients.arrow_forward
- Ammonia must be eliminated from wastewater before it can be reused, recycled, or discharged. Describe the three major steps that are used to convert harmful ammonia into a harmless derivative. Your answer must be specific and include any appropriate chemical formulas or names of chemical or biological reagents. Mention, when appropriate, the toxicity of any of the components or how their production can get in the way of efficiently eliminating ammonia from wastewater.arrow_forwardEnzyme b. Binding: Electrostatic Forces Substrates bind to receptors and enzymes through non-covalent interactions (intermolecular forces – IMF). Below is a cartoon of a part of a ligand or substrate interacting with an enzyme (blue line). There are two aspartic acid side chains of the enzyme that are interacting with the substrate (dashed lines). Identify the intermolecular forces. OH R Asp 125 -4----* H-o Asp25 Enzyme Active Sitearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY