
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
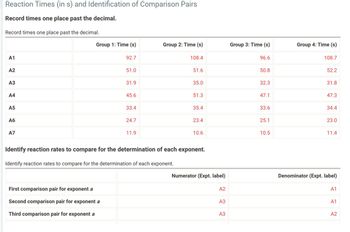
Transcribed Image Text:Reaction Times (in s) and Identification of Comparison Pairs
Record times one place past the decimal.
Record times one place past the decimal.
A1
A2
A3
A4
A5
A6
A7
Group 1: Time (s)
Group 2: Time (s)
Group 3: Time (s)
Group 4: Time (s)
92.7
108.4
96.6
108.7
51.0
51.6
50.8
52.2
31.9
35.0
32.3
31.8
45.6
51.3
47.1
47.3
33.4
35.4
33.6
34.4
24.7
23.4
25.1
23.0
11.9
10.6
10.5
11.4
Identify reaction rates to compare for the determination of each exponent.
Identify reaction rates to compare for the determination of each exponent.
First comparison pair for exponent a
Second comparison pair for exponent a
Third comparison pair for exponent a
Numerator (Expt. label)
Denominator (Expt. label)
A2
A1
A3
A1
A3
A2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The second law of thermodynamics has been called the arrow of time. Explain why this is so.arrow_forward. Hydrogen gas and chlorine gas in the presence of light react explosively to form hydrogen chloride H2(g)+Cl2(g)2HCl(g)The reaction is strongly exothermic. Would an increase in temperature for the system lend to favor or disfavor the production of hydrogen chloride?arrow_forwardFor the following reaction C(s)+2H2(g)CH4(g) K=0.26 at 1000C (3 significant figures). What is the equilibrium constant at 750C (3 significant figures)?arrow_forward
- Amoxicillin is an antibiotic packaged as a powder. When it is used to treat babies and small animals, the pharmacist or veterinarian must suspend it in water, so that it can be administered orally with a medicine dropper. The label says to dispose of unused suspension after 14 days. It also points out that refrigeration is required. In the context of this chapter, what is implied in the latter two statements?arrow_forwardFor the vaporization of one mole of bromine at 59C, determine (a) H (Table 8.2) (b) PV(c) Earrow_forwardThe following equation represents a reversible decomposition: CaCO3(s)CaO(s)+CO2(g) Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains?arrow_forward
- 5.12. True or false: If all the partial pressures of reactants and products drop by half, the value of Q drops by half. Give an example of a chemical reaction to support your answer.arrow_forwardFor the endothermic reaction AB(g)A(g)+B(g), the following represents a reaction container at two different temperatures. Which one (I or II) is at the lower temperature?arrow_forwardThe recycling of polymers represents only one industrial process that allows creating order in one location by creating greater disorder at some other location, often at a power plant. List three other industrial processes that must create disorder in the surroundings to generate the desired material.arrow_forward
- In Chapter 3, we discussed the conversion of biomass into biofuels. One important area of research associated with biofuels is the identification and development of suitable catalysts to increase the rate at which fuels can be produced. Do a web search to find an article describing biofuel catalysts. Then, write one or two sentences describing the reactions being catalyzed, and identify the catalyst as homogeneous or heterogeneous.arrow_forwardThe direct reaction of iron(III) oxide. Fe2O3, to give iron and oxygen gas is a nonspontaneous reaction; normally, iron combines with oxygen to give rust (the oxide). Yet we do change iron(III) oxide, as iron ore, into iron metal. How is this possible? Explain.arrow_forwardOld-fashioned smelling salts consist of ammonium carbonate, (NH4)2CO3. The reaction for the decomposition of ammonium carbonate (NH4)2CO3(s)2NH3(g)+CO(g)+H2O(g) is endothermic. Would the smell of ammonia increase or decrease as the temperature is increased?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning