
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
reaction rate equation on picture,
Determine, the unit value of k, the reaction constants for the various reaction orders
a. n=0
b. n=1
c. n=2
d. n=3
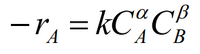
Transcribed Image Text:kC
A
´A
B
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1. Consider the set of liquid-phase series reactions 2A 3B →2C. The first reaction is second order, while the second reaction is first order. The rate constants at 40°C are k₁ = 0.3 L/mol/h and k₂ = 1 h¹, while EA₁ = 7 kcal/mol and EA2 = 0.20 kcal/mol. CA0 = 8 mol/L. a) Plot the concentrations of each species in a 100 L batch reactor at 40°C and 100°C over 10 hours (assume the reaction is liquid phase at both temperatures). b) What is the maximum concentration of species B at both temperatures? c) At what time do you get the maximum concentration of B at both temperatures?arrow_forwardc32 plz answer allarrow_forward10:27 Question 13 of 17 Submit For a particular reaction, K = 1.67. What can be said about this reaction? A) There are many more products than reactants in the equilibrium mixture. B) There are many more reactants than products in the equilibrium mixture. C) There are roughly equal amounts of reactants and products in the equilibrium mixture. D) The reaction has not yet reached equilibrium because K does not equal 1. Tap here or pull up for additional resourcesarrow_forward
- 4. The reaction A → B is nth order (were n = 1/2, 3/2, 2, 3, ·…) amd goes to completion to the right. Derive the expression for the half life in terms of k, n and [A]o.arrow_forwardDetermine the composite rates of reaction for all of the species in the following reaction sequence. А В --> С 2A --> D Where A --> B is 1st order, B --> A is 1st order, B --> C is 1st order, and 2A --> D is the second order reaction. respectively.arrow_forwardFor each of the rate laws below, what is the order of the reaction with respect to the hypothetical substances X, Y, and Z? What is the overall order? (For each answer, enter an exact number as an integer or decimal.) (a) ✗ Y rate = k [X][Y]² [Z] 2 Ꮓ Overall (b) -1 rate = k [X]0.5 [Y]1.5 [Z]¯1 X X 2 Y Z Overall [Z]³ (c) rate = k (X) 2 ☑> Y 2 Ꮓ Overallarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The