
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
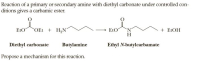
Transcribed Image Text:Reaction of a primary or secondary amine with diethyl carbonate under controlled con-
ditions gives a carbamic ester.
EtO
OEt + H,N
EtO
`N.
H
+ ELOH
Diethyl carbonate
Butylamine
Ethyl N-butylcarbamate
Propose a mechanism for this reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- CH3 OH CO₂H i) ii) = iv) v) x) Holyam E vi) vii) viii) ix) Match the carboxylic acid derivative with the correct description. (each used once) A HO سلمہ B H LOH OH Ja OH I F butanoic anhydride a lactone adipic acid cyclohexanecarboxylic acid succinic anhydride an a,ß-unsaturated acid an imine long cyclohexanecarboxylic anhydride 3-hexenoic acid a lactam J D G O NH OHarrow_forwardIdentify the product obtained in the hydrolysis of the following cyclic acid anhydride. CH₂ A) HOT B) C) НО D) HO H₂O, HCI (complete hydrolysis) w O OH + CH₂NH₂& H OH OH CH, SH,& CHÍNH CHÍNH CHarrow_forwardpropose a possible synthesis for the following:arrow_forward
- How would you prepare 3-phenyl-1-propanamine (C&H-CH₂CH₂CH₂NH₂) from C&H,CH₂CH₂CHO C HẠCH,CH,CHO Select the correct reagent for A. Ⓡ (CH,ANH, ơi (CH₂)NO > C HẠCH,CH,CH;NH, NaBH, CN CHÍNH, Ố NH₁ ?arrow_forwardH₂C 0 NH₂ B₂, NaOH 98 H₂C OH When an a-hydroxy amide is treated with try in aqueous NaOH under Hofmann rearrangement conditions, loss of CO, occurs and a chain-shortened aldehyde is formed. The mechanhum involves the following steps: Base abstracts an acidic amide proton, yielding amide anion The amide anion reacts with bromine in an e-substitution reaction to give N-bromoamide 2. Abstraction of the remaining amide proton by base gives a resonance-stabilized bromoamide anion Rearangement occurs to yield isocyanate Water adds to the isocyanate to yield carbamic acid Elimination of CD, yields carbinolamine Following proton transfer, expubion of ammonia yields the final product aldehyde Write out the mechanism on a separate sheet of paper, and then draw the structures of carbamic acid 5 and isocyanate 4. H 400, ANH, You do not have to consider stereochemistry. You do not have to explicitly draw Hatoms De not include lone pairs in your answer. They will not be considered in the grading…arrow_forwardSelect the appropriate reagent and TWO possible substrates for the following reductive amination.arrow_forward
- NOC XT Ph CH₂ Both primary and secondary amines add to a ß-unsaturated aldehydes and ketones to yield ß-amino aldehydes and ketones rather than the alternative imines. Under typical reaction conditions, both direct and conjugate modes of addition occur rapidly. But because the reactions are reversible, they generally proceed with thermodynamic control rather than kinetic control so the more stable conjugate addition product is often obtained to the complete exclusion of the less stable direct addition product. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H H CH3NH₂ CH3 H-A :A Ph NHCH3 вон CH3 :A H-A 8barrow_forwardPlease answer with explanations and make it easy to readarrow_forwardShow how to synthesize the following amines from the indicated starting materials byreductive amination. (±)-amphetamine 1-phenylpropan-2-onearrow_forward
- The compound 4-isobutylacetophenone is needed for the synthesis of ibupro- fen. Propose a synthesis of 4-isobutylacetophenone from benzene and any other necessary reagents. several steps COOH 4-Isobutylacetophenone Ibuprofen (racemic)arrow_forward1. Cetrizine is a nonsedating antihistamine. The first step in a synthesis of cetirizine involves the following Grignard reaction. Give the major product and propose a reasonable mechanism for the following reaction: H CI 1) 2) HCI, H₂O MgBr diethyl etherarrow_forwardWhat synthesis proposal can I use?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY