
Concept explainers
Please don't provide handwritten solution ......
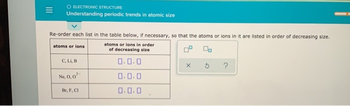
Atomic size is measure the distance between the nucleus and the outer most shell of the atom. Ionic radii refers as to the radii of ions in an crystals.
In the periodic table, when we go from left ot right in periods the atomic size decreases because the in coming electron goes to the same subshell. In the groups the atomic size is increases form the top to bottom because in coming electron goes to the new shell.
The ions are formed by the loss or gain of electrons the outermost shells of atoms. The ions formed by the loss of electrons are called as cations and the ions formed by the gain of electrons are called as anions.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

- Please anwer question and exlpain whyarrow_forwardAt a particular temperature, suppose that 24.4 g solute is added to 50.0 g water and, after mixing, 0.3 g of the solute remains undissolved. What is the solubility of this solute at this temperature in grams per 100 g of water? (Enter in standard notation with one digit after the decimal point) Type your answer......arrow_forward1. Develop a detailed separation scheme for the separation and determination of the percent composition of your sample which will be a mixture of NaCl, NH.CL, and sand. You are expected to use the properties of the components listed below and some of the techniques listed in the table in the pre-lab queries (and used in Separations I). Keep in mind that any chemistry student should be able to pick up your scheme, understand it, and use it to complete the separation and calculation of % composition. Place the scheme on a separate sheet of paper. Component Solubility (@25°C) Melting Point Hardness sodium chloride, NaCl 35 g/100 mL water 801°C soft ammonium chloride, NH&Cl 37 g/100 mL water sublimes 350°C soft sand, SIQ2 insoluble 1600°C hard Example of compounds in a container. Naci NH.CI sio2 Exploring the Chemical World, PGCC, 2003 4 RESULTS Show all calculations with units in this space.arrow_forward
- How many grams of CaCl2 (OR C a Cl2) would be required to prepare a 1.139 M solution with a volume of 531.00 mL? Do not type units with your answer.arrow_forwardPlease don't provide handwritten solution ..... The average human body contains 5,830g of blood. What mass of arsenic is present in the body if the amount in blood is 0.55 ppm?arrow_forwardHow do I calculate/ what is the equation for the % weight/weight when I have a mass in grams and a volume of titrant delivered? Unknown Chrloride mass 0.1173g and 35.47 ml.arrow_forward
- If sodium carbonate is the reactant in excess, carbonate ions will end up in the filtrate.Treatment of a sample of the filtrate with hydrochloric acid will result the production ofeffervescence. What physical evidence would indicate this reaction occurred?arrow_forwardPlease don't provide handwritten solution......arrow_forwardPOST-QUESTIONS 1. After a student dissolved 0.5 g of white solid in water, she tested the conductivity and determined that the solution did not conduct electricity. The student then concluded: "when the white solid dissolved it 'just disappeared' so some sort of error must have been made because all solids are ionic compounds and, therefore, are electrolytes." Comment on the student's conclusion. Is her statement a valid conclusion? If so, identify what is correct. If not, write a conclusion that would be correct.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





