
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
See image below. Can you please show me how to solve this?
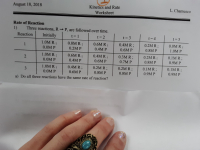
Transcribed Image Text:Rate of Reaction
1)
Three reactions, R P, are followed over time.
Reaction
Initially
t= 1
t= 2
t= 3
t= 4
t = 5
1.0M R;
0.8M R;
0.6M R;
0.4M R;
0.2M R;
0.0M R;
1
0.0M P
0.2M P
0.4M P
0.6M P
0.8M P
1.0M P
1.0M R;
0.6M R;
0.4M R;
0.3M R;
0.2M R;
0.1M R;
0.0M P
0.4M P
0.6M P
0.7M P
0.8M P
0.9M P
1.0M R;
0.4M R;
0.2M R;
0.2M R;
0.1M R;
0.1M R;
3.
0.0M P
0.6M P
0.8M P
0.8M P
0.9M P
0.9M P
a) Do all three reactions have the same rate of reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Consider the molecule of palmitic acid that is shown below. Draw a figure to show the behaviour of this molecule when it is added to an aqueous solution in high concentration. In your answer, describe what motivates the behaviour of palmitic acid in aqueous solution. HO,arrow_forward6. Suppose you have a mixture of water and your 2-bromo-2-methylbutane product in a separatory funnel. Use densities to predict which phase will be the top layer in the funnel. a. 2-bromo-2-methylbutane (organic phase) b. water (aqueous phase) c. there would only be one phase since the substances are misciblearrow_forwardL Moving to another question will save this response. Question 18 Which is not an important property of water? a. Solvency b. Adhesion c. Cohesion d. All of the above are important A Moving to another question will save this response. 15,213 FEB 25 Question 18 of 40> 2.5 points Question 18 of 40 tv Save Answer C A ssaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY