
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
infoPractice Pack
Question
infoPractice Pack
What is the activation energy of a reaction if it has the following rate constants?
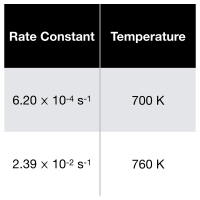
Transcribed Image Text:Rate Constant Temperature
6.20 x 10-4 s-1
700 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Includes step-by-step video
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why do reactions generally occur faster at high temperatures based on the collision theory? Choose all that apply High temperatures mean: - lower the activation energy requirement - high kinetic energy= greater number of collisions per time unit - increase solubility - high internal energy= more effective collisions by energy levels (activation energy)arrow_forwardWhat is the steady-state approximation? When is the steady state approximation a good approximation?arrow_forwardQuestion: What is the fundamental difference between kinetic stability and thermodynamic stability in chemical reactions, and how do they influence reaction rates?arrow_forward
- Kinetics Broadly speaking, the factors that influence chemical reaction rates may be summarised as follows.Chemical nature of reactants (e.g. is the chemical reaction thermodynamically “downhill” so that it can occur spontaneously at all?)Reactant Concentration (or pressure for a gas)Physical state of reactants (e.g. phase, or surface area of solids)Presence of catalystTemperature For each of the following observations, which one of the five factors is most important, and briefly explain how/why that is the case. (a) The ability to sustain aerobic exercise is limited by the amount of haemoglobin in the blood. (b) Non-starch polysaccharides are large complex carbohydrates, and an important source of dietary fibre. Their chemical energy content is similar to other carbohydrates like sucrose. These molecules do not undergo respiration via by human digestion, but some bacteria have specialised proteins that can process non-starch polysaccharides. (c) Potato is made edible by cooking it…arrow_forwardAt what point on a Potential Energy curve would you find the energy needed for an effective collision?arrow_forwardWhich of the following is true? Decreasing the activation energy decreases the reaction rate. Decreasing the activation energy increases the reaction rate. The activation energy of an exothermic reaction is negative. There is no relationship between activation energy and reaction rate.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY