
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Q16
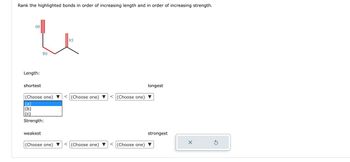
Transcribed Image Text:**Bond Length and Strength Comparison Exercise**
**Description:**
This exercise involves analyzing three distinct bonds, labeled (a), (b), and (c), highlighted in a molecular diagram.
**Diagram Explanation:**
- **Bond (a):** Located on the left with a triple line indicating a triple bond.
- **Bond (b):** Located in the middle with a single line indicating a single bond.
- **Bond (c):** Located on the right with a double line indicating a double bond.
**Task:**
- **Rank the highlighted bonds in order of increasing length:**
- From shortest to longest.
- Three dropdown menus are provided to select the order.
- **Rank the highlighted bonds in order of increasing strength:**
- From weakest to strongest.
- Three dropdown menus are provided for selection.
To complete the exercise, use the dropdown menus to correctly arrange the bonds based on their length and strength properties.
**Interaction Elements:**
- Dropdown menu options include (a), (b), and (c) for both length and strength rankings.
- Two control buttons:
- A reset button (circle with arrow) to start over.
- A submit button (check mark) to submit your choices.
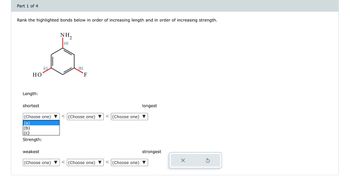
Transcribed Image Text:## Bond Analysis Activity
### Part 1 of 4
**Objective**: Rank the highlighted bonds below in order of increasing length and in order of increasing strength.
---
### Molecular Structure:
The diagram features a benzene ring with three different substituents:
- **NH₂ (Amino group)** labeled as (a)
- **F (Fluorine atom)** labeled as (b)
- **OH (Hydroxyl group)** labeled as (c)
Each substituent is attached to different carbon atoms of the benzene ring.
---
### Sorting Criteria:
#### Length:
- Arrange the bonds from shortest to longest.
1. **Option Dropdowns**: Select from options (a), (b), or (c) for each position in the sequence.
#### Strength:
- Arrange the bonds from weakest to strongest.
1. **Option Dropdowns**: Select from options (a), (b), or (c) for each position in the sequence.
### User Actions:
- Use the dropdown menus to select the appropriate ranking for each criterion.
Note: This activity helps understand chemical bonding characteristics based on the type of atoms and bonding involved.
---
**Tools**:
- Reset option available to clear selections and restart ranking.
Expert Solution
arrow_forward
Step 1: Interpretation of given problem
Given are organic structures.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete each row of the table below by filling in the missing prefix. 1 | Hz 3 10 Hz %3D 1 Hz 10 Hz -6 1 Hz 10 Hz %3D -1 1 Hz 10 Hzarrow_forward30 70- 60- 50- 40- 30 20 10 0₁ 4000 Ророжа Structue nd, C₂H₂1N 3500 иде Sp 3000 2500 18 +2-20 го- 2000 1800 hu 1600 Ф тро 1400 1200 1000 800 6 Н 6 Н 9 Н 600 Уarrow_forwardAns all parts plsarrow_forward
- 2+ d. 4H3O+ (aq) + 2Cl(aq) + MnO₂ (s) ⇒ Mn²+ (aq) + 6H₂O(1) + Cl₂ (9) Oke O Ke = = O Ke = 2+ [Mn²+ ][C1₂] [H3O+] * [CI-1² [Mn²+][H₂O][C1₂] 2+ [H³O+][CI¯]²[MnO₂2] [Mn²+ ] [H₂O1] [C1₂] 2+ [H3O+] * [CI-1²[MnO₂]arrow_forward6829Cu->0-1beta+?arrow_forwardWhich of the following values for R2 shows the strongest correlation between the X and Y values in a data set? 0.0184 0.0047 0.500 0.981 0.9678arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY