
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:AINVauv
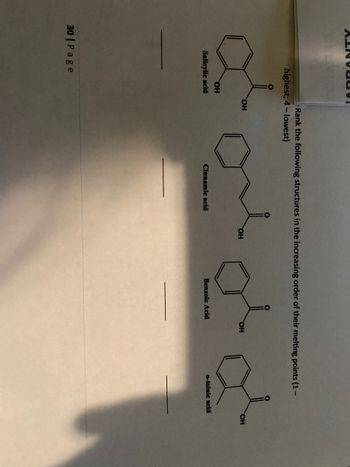
Rank the following structures in the increasing order of their melting points (1-
highest; 4-lowest)
OH
Salicylic acid
30 | Page
OH
Cinnamic acid
OH
Benzoic Acid
OH
o-toluic acid
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 21) N,N-dimethyl-2-propanamine Draw the skeletal structure of the following compoundarrow_forwardDoarrow_forwardAny structure for the benzene must account for the following facts, except; a. None of the above b. It is planar c. All C-C bond lengths are equal d. It has three alternating π bonds around the benzene ring e. It contains a six-membered ring and three additional degrees of unsaturationarrow_forward
- 5. Which of the following molecules is aromatic or anti-aromatic? Support your claim with an MO diagram and checking the molecule against the rules of aromaticity. CHarrow_forwardIs this molecule aromatic? Why doesn't the pi bond in the benzene ring on the left side not count torward aromaticity?arrow_forwardWhich of the attached cyclic molecules are meso compounds?arrow_forward
- Please answer very soon will give rating surelyarrow_forwardthe drawing utility. Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of C6H13Br and two stereogenic centers. Indicate chirality by using wedge and hashed wedge notation. Lone pairs do not need to be shown. edit structure ...arrow_forwardPlease provide the hybridization for each carbon.arrow_forward
- verse osis Consider the structures of the carbocations formed by ortho attack of the electrophile, *NO₂, on the given starting material. NH2 Part: 0/2 Part 1 of 2 + Draw all resonance structures for the carbocation formed by ortho attack of the electrophile "NO, on the given starting material. If applicable, include the resonance structure in which π bond electrons "move" to the more electronegative atom as a lone pair. Be sure to include all charges and relevant lone pairs. 00 al. Ar B Karrow_forwardGive clear detailed Solution with explanation needed..don't give Handwritten answer...arrow_forwardHow many sp2 hybridized carbons are there? How many sp3 hybridized carbons are there? Please assign R or S to the carbon marked with the arrow labeled with an “A” Please assign R or S to the carbon marked with the arrow labeled with a “B”arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY