
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Rank the following compound in terms of increasing predicted boiling points. Explain your answer.
Compounds: Benzene Ring, phenol, dichlorobenzene, Phenyl
Expert Solution
arrow_forward
Step 1
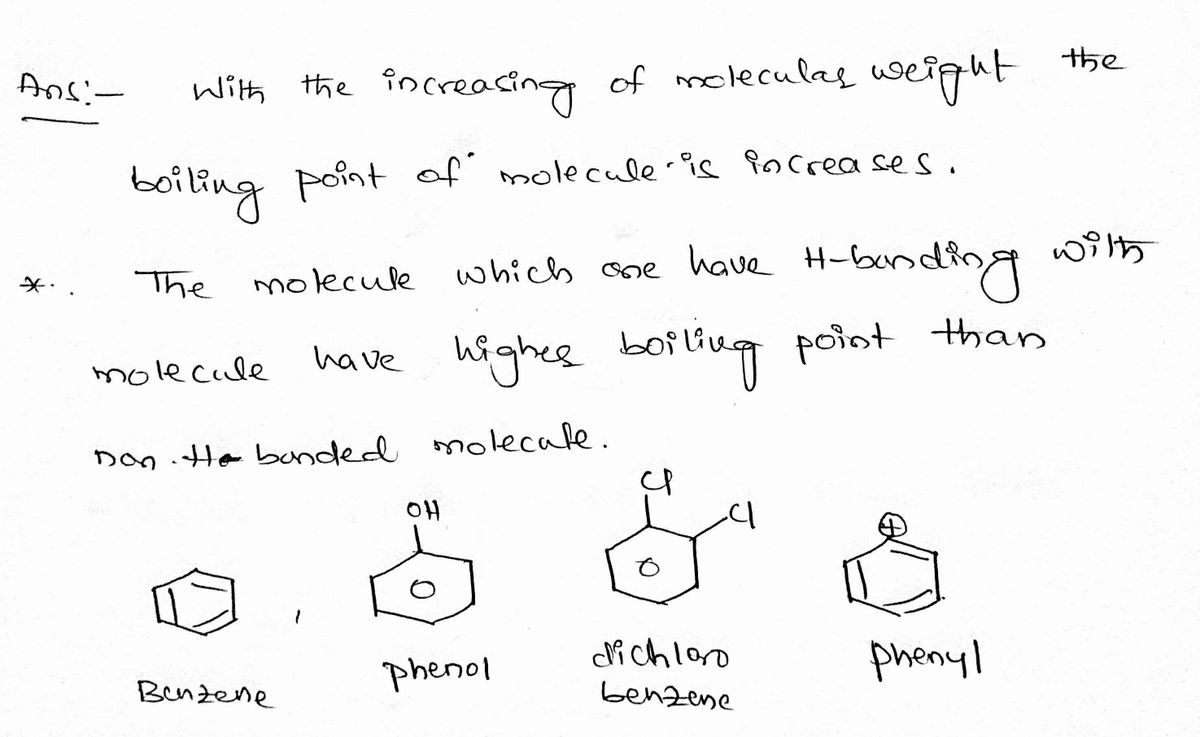
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethers can be classified as open chain or cyclic. Open chain ethers can contain rings. What is the structural difference between open chain ethers that contain rings and cyclic ethers?arrow_forwardIn this task you will compare the chemical properties of alcohols and carbonyl compounds. Complete the table below. Aliphatic compound Homologous series Type of reaction(s) addition/substitution/redox Propan-1-ol Propan-2-ol 2-methylpropan-2-ol Ethanal Propanone 2. Describe the similarities and differences in the chemical reactions between alcohols and carbonyl compounds. Include reaction equations.arrow_forwardGiven the reaction: 4 Fe(s) + 3O2(g) → 2 Fe203(s) A H= -1652 kJ, what is the enthalpy change when 2.00 moles of Fe203 are produced? O 1652 kJ O - 1652 kJ O 826 kJ O - 826 kJarrow_forward
- Look at the structures of aniline, N-methyl aniline and Triethylamine rank these compounds in order of increasing solubility in water.arrow_forwardPlease help in answering the organic chemistry questions given below. Explanations are welcome.arrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilid aromatic substitution reactions are excluded?arrow_forward
- I know there is: 1. Carboxylic group 2. Ester. 3. Ortho-disubstituted phenyl. But my answer is still partial.arrow_forward1. Write a reaction showing how the compound ethanoic propanoic anhydride is prepared. Draw and name all constituional isomers of a compound having the molecular formula C4H&O2. 2.arrow_forwardThis CAN NOT be hand-drawn. Please type out all explanations. Please utilize a computer program to illustrate any examples! Thank you.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY