
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
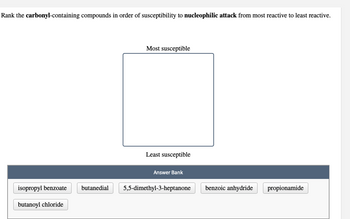
Transcribed Image Text:Rank the carbonyl-containing compounds in order of susceptibility to nucleophilic attack from most reactive to least reactive.
isopropyl benzoate
butanoyl chloride
butanedial
Most susceptible
Least susceptible
Answer Bank
5,5-dimethyl-3-heptanone
benzoic anhydride
propionamide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the IUPAC name for the following compound. CH3-CH;-CH,-C-0-CH-CH, ethyl propanoate 4-hexanal ethyl propyl ether ethyl butanoate hexanoic acid « Previousarrow_forwardReaction with which of the following compounds in an acetylide reaction would lengthen the carbon chain by one carbon and add a primary alcohol? ethanal formaldehyde epoxide acetone carbonic acidarrow_forwardDraw the products obtained by reacting a sulfonitric mixture with: 4-nitrophenol, 2,4-dinitrophenol, phenol, 4-methoxyphenol, 4-chlorophenol.arrow_forward
- Draw the reaction of the Grignard reagent interacting water and the product produced. bromobenzene Br MgBr Mg ether HCI phenyl magnesium bromide (Grignard reagent) OH off triphenyl methanolarrow_forward2. There are two methods to convert an alkyl halide into a carboxylic acid containing one additional carbon atom: R-X R-X NaCN CH3CI Mg R-CN Br R-MgX H3O+ 1) CO₂ 2) H3O+ (CH3)3CCI i Depending on the structure of the alkyl halide, one or both of these methods may be used. For each alkyl halide shown, write out a stepwise mechanism that converts it to a carboxylic acid. If both methods work, draw both routes. If one method cannot be used, state why it cannot. HO R OH AiOH R OH Brarrow_forwardcan I get an explanation on how to do these? Thank youarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY