
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please explain carbocations and the increasing stability
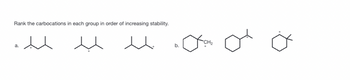
Transcribed Image Text:Rank the carbocations in each group in order of increasing stability.
从从从
a.
Ja
Expert Solution
arrow_forward
Step 1: Interpretation of given problem
Given compounds are carbocations.
Carbocation is species in which carbon carries positive charge.
Primary carbon is carbon that has another one carbon attached.
Secondary carbon is carbon that has another two carbons attached.
Tertiary carbon is carbon that has another three carbon attached.
Quaternary carbon is carbon that has another four carbons attached.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 20. In the IUPAC name for the following compound, the -Br group is located on carbon number_? CH;CHCH=CH, Br a. 1 b. 2 С. 3 d. 4arrow_forward12. Which of the following carbocations is the least stable one? 13. The heats of complete hydrogenation of the following three hydrocarbons are 105, 116, 275 and 292 (in kJ/mol). Which hydrocarbon does feature the heat of hydrogenation of 292 kJ/mol?arrow_forward1. Which term best describes the alkene below? a. cis only b. trans only c. both terms may be used d. neither cis nor transarrow_forward
- Classify each carbocation as primary, secondary, or tertiary. a. b. 人 C. d.arrow_forwardWhich structure is a meso compound? A. В. С. D. CH3 CH 3 CH3 CH 3 H--C1 H--C1 H--C1 H--C1 cl-- - CH3 Cl--H H--C1 Cl--H CH 3 CH2CH3 CH2CH3arrow_forward4. Syn and anti addition can occur with alkenes. 1. Describe syn and anti addition. b. Give an example of syn addition to an alkene and explain why it is syn. c. Give an example of anti addition to an alkene and explain why it is anti. 5. The following reactions shows the dehydration of 2-methylcyclohexanol. Why is 1-methylcyclohexene the major product? X OH H3PO4 84% 16%arrow_forward
- What is the IUPAC name for the following compound? A. (2E, 4Z)-2,4-hexadiene B. (2E, 4Z)-1,4-dimethyl-1,3-butadiene (2Z, 4Z)-1,4-dimethyl-1,3-butadiene (2Z, 4Z)-2,4-hexadiene ABCD C. D. MacBook Airarrow_forwardGive the total number of stereoisomers of 2,3- dibromobutane. A. none B. 2 C. 3 D. 4 E. 5arrow_forwardName the following molecules, using IUPAC naming conventions. Indicate all stereochemistry in the namearrow_forward
- What is hyperconjugation? How is hyperconjugation involved in the stabilisation of carbocations?arrow_forwardThe two alkenes below react with HI at different rates. CH3CH=CHCH3 and CH₂=CH₂ Draw the structural formula of the MAJOR product formed by the alkene having the HIGHER reaction rate. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. TAYY [ ] در ChemDoodle Ⓡarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY