
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Bb.34.
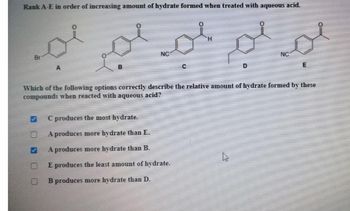
Transcribed Image Text:Rank A-E in order of increasing amount of hydrate formed when treated with aqueous acid.
لو لو لم لم اجد
Br
NC
✔
H
C produces the most hydrate.
A produces more hydrate than E.
A produces more hydrate than B.
E produces the least amount of hydrate.
B produces more hydrate than D.
D
Which of the following options correctly describe the relative amount of hydrate formed by these
compounds when reacted with aqueous acid?
پائے
NC
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. To prepare 250 ml of 0.35 M NaOH using 8 M NaOH, you dilute ------ml of ------M with water to 250 ml and shake. * O 10, 0.35 11, 0.35 O 10,8 11,8 ✓ 12,8arrow_forwardD D D D Δ ΔΔΔΙΔ to e quivalents 2 σκελί Convert 1.87 mol50, 2007 31. Eqarrow_forwardDetermine the mass of the water in the sample based on the following data to teo decimal places. Mass of crucible and cover- 50.02 Mass of crucible, cover and sample before heating-51.04 Mass of crucible, cover and sample after heating-50.86arrow_forward
- 5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution ALPHA is diluted to 25.00 mL, resulting in solution BETA. 10.00 mL of solution BETA is then diluted to 25.00 mL, producing solution GAMMA. dilution factor for ALPHA from stock solution = 0.167 dilution factor for BETA from ALPHA solution = 0.0476 part c and d?arrow_forwardSay you have a stock of 15% NaCl and need a reaction concentration of 1% in a total reaction volume of 25ul. How many ul of the 15% NaCl stock would you need to add to the reaction tube?arrow_forwardArrange these phenolic compounds in order of increasing acidity. Least acidic Most acidic Answer Bankarrow_forward
- A solution is prepared by dissolving 23.4 g of CaCl2 (MW = 110.98 g/mol) in 355 mL of water. The density of the resulting solution is 1.05 g/mL. The concentration of CaCl2 in this solution is ________ molar.arrow_forwardYou have a sulution containing 135.2a f diselved KBrin 2.3L of waiter. what volume of this: Solution in ml, would to make 5 Lofa O. 10 M KB- Soluhon? you usearrow_forward11. A mixture was found to contain 4.36 g of NazCO3, 6.21g of S403 and 1.89 g of LI2Se: What is the percentage of Lithium selenium?arrow_forward
- A physician prescribed dimenhydrinate (Dramamine) 50 mg q4-6 h. p.o. as an antihistamine. The drug is available in 0.2 g tablets. Calculate how many tablets are to be given.arrow_forwardTTTTT 3. Jeanette wants to prepare a 0.125 mol/L solution of sucrose C12H22O11. The desired volume Jeanette wants to make is 50 ml. Formula Mass= Volume (L)= Moles Sucrose = Mass Sucrose= 0.050 L of water (solvent) ? Mass of Sucrose Formula Mass= Volume (L) - Moles LI SO Mass Li₂SO. = Mass of 0.050 L of 0.125 mol/L Sucrose 4. Grover wants to prepare a 6.00 mol/L solution of Li,SO.. The desired volume Grover wants to make is 500 ml. Li-SO Show work in this space SLIVEWORKSHEETS Show work in this spacearrow_forwards.) Br DMF(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY