
Advanced Engineering Mathematics
10th Edition
ISBN: 9780470458365
Author: Erwin Kreyszig
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Question
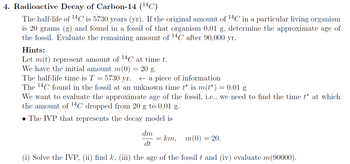
Transcribed Image Text:4. Radioactive Decay of Carbon-14 (¹4C)
The half-life of ¹4C is 5730 years (yr). If the original amount of ¹4C in a particular living organism
is 20 grams (g) and found in a fossil of that organism 0.01 g, determine the approximate age of
the fossil. Evaluate the remaining amount of ¹4C after 90,000 yr.
Hints:
Let m(t) represent amount of 14C at time t.
We have the initial amount m(0)
20 g.
The half-life time is T = - 5730 yr.
a piece of information
The 14C found in the fossil at an unknown time t* is m(t*) = 0.01 g
We want to evaluate the approximate age of the fossil, i.e., we need to find the time t* at which
the amount of ¹4C dropped from 20 g to 0.01 g.
• The IVP that represents the decay model is
dm
dt
m(0) = 20.
(i) Solve the IVP, (ii) find k, (iii) the age of the fossil t and (iv) evaluate m(90000).
= km,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- 2arrow_forwardFind Half life: U1= 2110 U2= 2159 X1= 9855 X2= 4315 t (days) = 56 please find value of T1/2arrow_forwardCarbon-14 sa radioactive element with a half-afe of about 5,730 years. Carbon-14 is said to decay exponentialy. The decay rate is 0.000121. We start with one gram of cartbon- 14. We are interested in the time (years) it takes to decay carbon-14. Find the percentage of carben-14 lasting longer than 15,000 years. (0) Sketch the graph, and shade the area of interest. G.00014 0.8 0.00012 0.00010 0.6 0.00008 0.00000 04 0.00004 0.00002 0.2 s000 10000 15000 20000 3000 10000 13000 20000 1.0 0.00014 0.00012 0.8 0.00010 0.6 0.00008 0.00006 04 0.00004 0.00002 02 5000 10 000 15000 20 000 5000 10 000 15 000 20 000 (b) Find the probability. (Round your answer to four decimal places.) P15,000) -arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated
Advanced Engineering MathematicsAdvanced MathISBN:9780470458365Author:Erwin KreyszigPublisher:Wiley, John & Sons, Incorporated Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education
Numerical Methods for EngineersAdvanced MathISBN:9780073397924Author:Steven C. Chapra Dr., Raymond P. CanalePublisher:McGraw-Hill Education Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY
Introductory Mathematics for Engineering Applicat...Advanced MathISBN:9781118141809Author:Nathan KlingbeilPublisher:WILEY Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,
Mathematics For Machine TechnologyAdvanced MathISBN:9781337798310Author:Peterson, John.Publisher:Cengage Learning,


Advanced Engineering Mathematics
Advanced Math
ISBN:9780470458365
Author:Erwin Kreyszig
Publisher:Wiley, John & Sons, Incorporated

Numerical Methods for Engineers
Advanced Math
ISBN:9780073397924
Author:Steven C. Chapra Dr., Raymond P. Canale
Publisher:McGraw-Hill Education

Introductory Mathematics for Engineering Applicat...
Advanced Math
ISBN:9781118141809
Author:Nathan Klingbeil
Publisher:WILEY

Mathematics For Machine Technology
Advanced Math
ISBN:9781337798310
Author:Peterson, John.
Publisher:Cengage Learning,

