
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help please
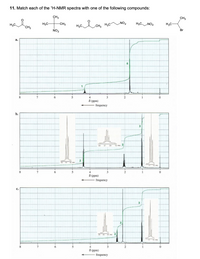
Transcribed Image Text:11. Match each of the 'H-NMR spectra with one of the following compounds:
ÇH3
CH3
H3C
H3C-
-CH3
H3C
NO2
H3CNO2
H3C
H3C.
CH3
NO2
Br
a.
4
8 (ppm)
frequency
b.
20
8
6.
4
3
8 (ppm)
frequency
с.
6.
4
8 (ppm)
frequency
Expert Solution
arrow_forward
Step 1
A graph is for the compound no 6 .
(CH3)2CHBr , here Both the CH3 are equivalent and thus give doublett with respect to the CH proton , where as CH protons give multiplet wrt both methyl group .
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Do not give handwriting solution.arrow_forwardMatch each alcohol below to its proper category (primary, secondary or tertiary) CH3 ОНarrow_forwardWrite as many accurate and relevant statements about the following visual prompt as you can think of. No less than 8 statements. Drawings with descriptions of their meanings can be considered a response too.arrow_forward
- Concentration (M) 1 0.8 0.6 0.4 0.2 0 0 1st attempt 10 y = -0.0111x + 1.0362 20 30 Time (minutes) What is the y-intercept of this graph? 40 50 60arrow_forwardHP TrueVision HD 4 Texas Workfor Mộc Homework × W. College Physic mical App AN Careers eagleonline.hccs.edu/courses/188795/assignments/3601808 valnabie diler reD 4 dl 10.1Tam Constants | Periodic Table Part O Complete the fourth row. Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic. Express your answer using two decimal places. Acidic or [H+] [OH-] pH pOH basic? 7.4x10-3 ΑΣφ 3.3x10-10 M pH 8.25 5.73 Is to fa ho II 10 insertarrow_forwardIs something wrong here?arrow_forward
- 15 16 17 18 19 20 21 22 23 24 25 26 27 An aqueous solution at 25 °C has a OH concentration of 1. x 10 "M. Calculate the H,O' concentration. Be sure your answer has 1 significant digits. -10 M dh Submit Assignment Continue MacBook Air F12 F9 F10 888 O00 FB 30 F7 F5 F6 esc F4 F2 F3 F1 & @ # $ %3D 4 5 7 8 9 2 3arrow_forwardPlease don't provide handwriting solutionarrow_forwardPost-Laboratory Questions 1) An extra strength antacid tablet contains 750 mg of active ingredient, CaCC 22.25 mL of HC1 (aq) to neutralize the tablet, how strong is the acid? 2 HC1 (aq) + CaCO3 (aq) → CaCl,(aq) + HOH (1) + CO2 (arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY