
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
The distillation column shown in Fig is used todistill a binary mixture. Symbols x,y, z denote mole fractions of the more volatile component, while B,D,R,andFrepresent molar flow rates. It is desired to control distillate composition y despite disturbances in feed flow rate F. All flowrates can be measured and manipulated with the exception of F, which can only be measured. A composition analyzer provides measurements ofy.(a)Propose a feedback control method and sketch the schematic diagram.(b)Suggest a feedforward control method and sketch theschematic diagram.
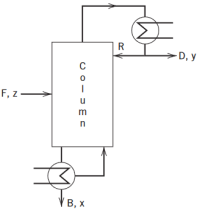
Transcribed Image Text:R
-D, у
F, z-
VB, x
U o- = E c
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 7 A flammable liquid is being transferred from a road tanker to a bulk storage tank in the tank farm. What control measures would help reduce the risk of vapour ignition due to static electricity.?arrow_forwardConsider the following endothermic reactions occur in a reactor. A →→→→B C Where r₁ and r₂ are the reaction rates in mol/s: r₁=3.86x10°CAV, exp(-5033/T) r2=1.86x10¹³ CBV, exp(-10065/T) CA and CB are the concentration of component A and B, respectively. T is the reaction temperature in kelvin. Assume feed contains only component A, and neglect the utility cost. Process parameters: C-1.gmol/l (concentration of A in feed) F-10 L/s (volumetric feed rate) V V=100 L (volume of reactor) Optimizer RSP (FC) Steam Feed- F Fy 40 RSP TC 0 A-B-C Product C₂Cp Ccarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- 1. A feed of equimolar (50%) binary system mixture of (A&B components) is processed in a distillation column to achieve a mole fraction of A at the top of the column XD=0.9, while its mole fraction at the bottom product is 0.05. Component A is the more volatile component in this mixture. a. determine the number of required stages to achieve the above-mentioned purity. b. If you use tray column, how many trays are required if the tray efficiency is 85% Please use the following data to solve the problem. Please use a graph paper to draw the diagram. You may download graph paper from google if you do not have. Alpha (α) 3 Rmin 1.2 q 0.5 R=1.5*Rminarrow_forwardWhich among the following is FALSE for distillation equipment? a. A flash drum operates at lower pressures to vaporize the more volatile component. b. Partial condensers operate with vapor-liquid equilibrium. c. Total condensers yield reflux and distillate streams that have composition similar to the incoming saturated vapor. d. Partial reboilers yield a liquid stream that is recycled back to the distillation column.arrow_forwardMulti correct answers. Please select all correct answers: which of the following strategy could be used to increase the mass transfer rate inside an aerated stirred fermenter? (oxygen needs to transfer from the bubbles (gas phase) to the media which is a liquid phase (KL(Cs-C) )). Question options: Decrease bubble size Increase air flow rate Increase stirring rate Increase bubble sizearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The