
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:%01 ll. lin. e
quiz 1.pdf >
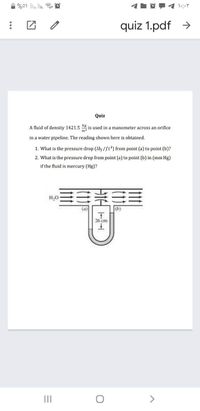
Quiz
A fluid of density 1421.5
is used in a manometer across an orifice
in a water pipeline. The reading shown here is obtained.
1. What is the pressure drop (Ib; /ft) from point (a) to point (b)?
2. What is the pressure drop from point (a) to point (b) in (mm Hg)
if the fluid is mercury (Hg)?
(a)
(b)
26 cm
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 5arrow_forwardGiven information is attached. A. Neglect friction and calculate the velocity at point 2. (Velocity in m/s) B. If the pipe diameter at point 2 is 7.00 cm, what is the diameter at point 1? (Diameter in cm)arrow_forwardA pipeline conveys benzene from one point to the other. A gauge attached to line reads 150kPa. It is desired to check the gauge reading with benzene –over-mercury U-tube manometer.Determine the expected height difference of the mercury in the manometer; if on one endthe manometer is open to the atmosphere and on the other end where the gauge pressure isconnected there is a 3 cm height of benzene above the mercury meniscus. (benzene densityis 876 kg/m3 and mercury 13600 kg/m3)arrow_forward
- 10.33 ft³/min of a liquid with density (SG=1.84) is pumped 45 feet uphill. At the inlet, the pipe inner diameter is 3 in and the liquid pressure is 18 psia. At the outlet, the pipe inner diameter is 2 in and the liquid pressure is 40 psia. The friction loss in the pipe is 11.0 ft lbf/lbm- Determine the work required (hp) to pump the liquid. (Hint: gc will be helpful in this problem.)arrow_forwardالعنوان > TIONTICWUTK A liquid with sp. gr. = 1.12 flows through a pipe with velocity 1 m/s. The diameter of the pipe is 4 in. Calculate: (i) The mass flowrate (kg/s), and (Ibm/min) (ii) The volumetric flowrate (m³/s), and (ft³/min) Ans.: (1) 9.08 Kg 1198.5 lb/min (it) 0.0081 m³/s. 17.15ft³/min Solve Ans: ۱/۱ で CICarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The