
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
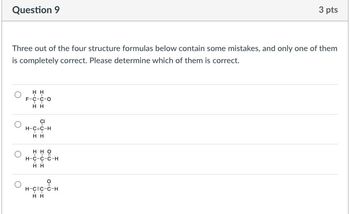
Transcribed Image Text:Question 9
3 pts
Three out of the four structure formulas below contain some mistakes, and only one of them
is completely correct. Please determine which of them is correct.
HH
F-C-C-O
TT
HH
CI
H-C=C-H
H H
H-C-C-C-H
HH
о
H-C=C-C-H
HH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 6 images

Knowledge Booster
Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardW AutoSave Off 周り File Home Insert Draw Assignment 3-1 Saved to this PC ✓ Design Layout References Mailings Review A A Aa Times New Roman ✓ 12 ✓ Paste BIU ab x² A ✓ く Clipboard Гу Page 5 of 10 1 76°F Clear 254 words ㄈㄨˋ Font Search Christiana Taylor CT Comments Editing ✓ Share View Help Find P ✓ ✓ = = ↓¶ Normal No Spacing Heading Heading 2 ГУ Paragraph ☑ Styles 3. Determine and draw the structure corresponding to the spectral information provided below. As you make this determination, complete the table below. Explain what clues you used to determine the structure. S C5H1002 Signal б ABC -4.3 -2.0 с -1.3 PPM Integration Multiplicity m (multiplet/septet) S d 叹 Text Predictions: On Accessibility: Investigate 2 19 Q Search W ✓ Replace Select ▾ Dictate Editor Add-ins Editing Voice Editor Add-ins > | + 88% Focus 8:01 PM 4/2/2024arrow_forward10arrow_forward
- MasteringChemistry .edu/webapps/assessment/take/launch.jsp?course_assessment_id%3_1575667_1&course_id%3_592130_1&content_id%3D_39106439 18 Up pe Maps M Gmail YouTube O Maps * Question Completion Status: QUESTION 4 A student takes 92 ml of a 0.0245 molar KOH solution and dilutes it to a final volume of 4,314.5. What is the pH of that resultant dilute solution? (report pH to two decimal places and include NO units in your answer) QUESTION 5 A student is told by their chemistry professor that he thinks he remembers that the ion product constant for water (Kw) has a value of 1.51x10-14 provided by their chemistry professor from his questionable memory, what is the calculated pH of water at 40 °C ? (Enter your number with 2 decimal places and do NOT include "pH" as a unit in your answer.) when water has the same temperature as that of a human running a hot fever of 40 °C. Based only on this information QUESTION 6 Given the information for equilibria (1) and (2) below, find the equilibrium…arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardPlease use the compounds I-L from image one and fill in the columns from image 2arrow_forward
- keAssignment/takeCovalentActivity.do?locator-assignment-take M) red req req eq eq eq eq eq eq Use the References to access important values if needed for this question. A compound is found to contain 42.88 % carbon and 57.12 % oxygen by mass. What is the empirical formula for this compound? To answer the question, enter the elements in the order presented above. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY