
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
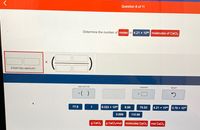
Transcribed Image Text:Question 8 of 11
Determine the number of moles in 4.21 x 1023 molecules of CaCl2
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
77.6
1
6.022 x 1023
6.99
75.53
4.21 x 1023
3.79 x 1021
0.699
110.98
g CaClz
g CaClz/mol
molecules CaCl2
mol CaCl2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HELP me pleasearrow_forwardSTARTING AMOUNT X How many moles of propylene (C3H6) are in 25.0 ADD FACTOR 1.01 1 4.15 x 10-23 25.0 molecules C3H6 12.01 6.022 x 1023 mol C3H6 ANSWER 36.03 42.09 g C3H6/mol g of the substance? 0.694 g C3H6 RESET 0.594 DDarrow_forwardPlease just answer the first questionarrow_forward
- Convert 1.312 mol of NaHCO3 to gramsarrow_forwardWhat mass in grams are in 5.32 x 1022molecules of CO2 ? STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 5.32 x 1022 6.022 x 1023 44.01 3.89 2.01 x 10-3 1.41 x 1048 28.01 0.0883 molecules CO2 g CO2/mol g CO2 mol CO2arrow_forwardNeed Help to solve or check if my answers are correct. Thanksarrow_forward
- Percentage to Compounds to formulas Question Part 1: A compound is found to contain 26.73 % phosphorus , 12.09 % nitrogen , and 61.18 % chlorine by mass. The empirical formula for this compound is_________________Question Part 2: The molar mass for this compound is 115.9 g/mol. The molecular formula for this compound is_______________arrow_forwardIn this question below, what is the starting unit? How many molecules of carbon monoxide (CO) are needed to react with excess iron (III) oxide (Fe203) to produce 11.6 g of iron (Fe)? O g Fe203 O g co O g Fe O g Co2 O mole Fe203 O mol CO O mol Fe O mole CO2 Oparticles Fe203 O molecules cO Olatoms Fe molecules CO2arrow_forwardconvert 698N to mol 4.9mol 4mol 9.4mol 3.9mol none of the abovearrow_forward
- O STARTING AMOUNT esc X # According to the balanced reaction below, calculate the moles of NH3 that form when mol of N₂H4 completely reacts ADD FACTOR X () 32.06 3.2 mol NH3 $ 4.2 1 % 3 g N₂H4 Question 24 OT 38 of N₂H4(1)→ - 6.022 x 1023 4.2 mol N₂ 3 4 NH3(g) + N₂(g) 6 5.6 ANSWER g N₂ 28.02 O 7 16.8 17.04 mol N₂H4 RESET 2 g NH3 4 Feb 23arrow_forwardMOLAR VIEW T03/S11 If a sample of SO3 is found to contain 5.29 mol of S atoms how many moles of O atoms are present? Molar Mass (g/mol) SO3 S atom O atom Avogadro's No. (mol"1) 6.022x1023 80.058 32.066 15.999 the tolerance is +/-2%arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY