
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
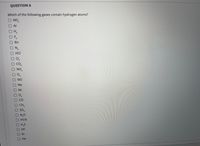
Transcribed Image Text:QUESTION 6
Which of the following gases contain hydrogen atoms?
NO2
O Ar
OH,
OF,
Rn
ON,
OHCI
O C,
O CO,
O NH,
2.
O NO
O Ne
O Xe
OCO
O CH,
O So,
ONO
OHCN
OH.S
HF
Kr
Не
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ||| V What are the molecular and empirical chemical formulas of a compound made up of these molecules? H H H H | | —C—C—CO-H O ATOMS, IONS AND MOLECULES Understanding the difference between a molecular and empiric... esc H—Ö- H-Ö-c 1 HTCI I H molecular formula: empirical formula: Q Explanation 2 Check W -CIH Н 0 0 #3 E The lines stand for chemical bonds between the atoms. You can ignore the dots you'll learn about them later. C X > $ 4 R S Costa do 5 % 6 MacBook Pro T A 6 G I Y 7 2022 McGraw Hill LLC. All Rights Rese H You 8 U Jarrow_forwardQUESTION 5 Which one of these compounds is an ionic compound? O a. N2 O b. CO2 O c. Na20 O d. CCI4arrow_forwardWhat other chemical would follow the +2/ -1 rule shown here? (Hint: look at the common charges of each of the elements, then make sure they balance properly.) O BeO2 O Na20 Srl2 O BażFarrow_forward
- Which of the following gases consist of molecules containing three or more atoms?? O Rn ON,O O Ar OF, O Kr O CH ON O NH, O Ne O HF NO, O He CO. Xe CO so, 2. HCI NO O HCN OH,Sarrow_forwardWrite Chemical Name and determine if it Is A. lanic compound B. Covalent compound C. Acid D. Oxyacid E. Bases 1. Mrig C 2. Hy SiOz 3. Irtezarrow_forwarded Complete the following for the compound ammonium phosphate. formula = (NH4)3PO4 atom nitrogen hydrogen phosphorus oxygen Submit Answer number in formula important values if needed for this question Retry Entire Group 9 more group attempts remainingarrow_forward
- b Answered: Question 52 of 65 V X 101 Chem101 G chemical formula for the bisulfa X C Question 46 Of 46 Submit Wha X + app.101edu.co M Apps G M Gmail YouTube Маps a AMAZON Translate Gflights USCIS b BATERBLY C CHEGG > KATAPULK CUBA SUPERMARKET23 Reading List Question 46 of 65 Submit Write the chemical formula for the bisulfate ion 4- 2+ 3+ 4+ 1 4 6. 7 8. 9. O3 (s) (1) (g) (aq) Na H. S Ph Si Reset x H,O Delete + LO 2. 4. 3. 2. 2.arrow_forwardWhat is the formula for the compound formed from Na1+ out the formula in a line, for example Na2CO3 (no subscripts, formulas are case and 02- ions? Please write sensitive!).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY