
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
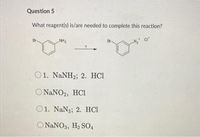
Transcribed Image Text:Question 5
What reagent(s) is/are needed to complete this reaction?
Br.
NH2
Br.
N2 Cr
O1. NaNH2; 2. HC1
O NANO2, HCi
O1. NaN3; 2. HCl
O NANO3, H2 S04
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What oxidizer is used to create the chemical known as "piranha" in the semiconductor industry?a. superoxide of potassium b. hydrogen peroxidea. peroxydisulfuric acid b. peroxydisulfuric acid c. peroxydissodium persulfate d. sodium persulfatearrow_forward10.) For the reaction:A + B + C ↓ productsthe following data was obtained. Complete the table. trial # [A]0 (M) [B]0 (M) [C]0 (M) initial rate mole/L.s (M/s) 1 0.21 0.11 0.10 7.01x10-6 2 0.21 0.11 0.20 1.40x10-5 3 0.21 0.33 0.10 7.01x10-6 4 0.42 0.11 0.10 2.80x10-5 5 0.84 0.11 0.10 6 0.21 0.11 3.50x10-6 7 0.11 0.10 1.40x10-5arrow_forwardPrepare a material flowsheet similar to Figure 6.1 (shown below) for production of 2400 lb-mol/hr of Vinyl Chloride (VC) based on the reaction path #5 with the operating parameters provided below. You MUST show the Molar flow rates of ALL compounds in ALL streams in your flowsheet. Reaction Path #5. Balanced process for Chlorination of Ethylene C2H4 + Cl2--> C2H4CI2 (C) Ileat Liberated Ileat Absorbed y Reactinn 150 x 10 Blutr duriag Reaction = 52 x 10 Bluhr C2H4 + 2HCI + 1/2 O2--> C2H4CI2 + H2O 2C2H4CI2 --> 2C;H3CI + 2HCI (E) (D) 58.300 Ibhr 2C2H4 + Cl2 +1/2 O2 --> 2C2H3CI + H2O (C) 100% Conv. of Cl2 at 80°C and 150 kPa with 15% (G) Direct Chlorination YƯ C, 15 atm Pyrolysis Sorc +CH,CI- 26 ata HCI CH.CI, CAL 11.3,4KI hr 158,I Ihyhr CH,CI, C1,CI 4,900 Ihhr CH, -a CH,CI, excess C2H4 CH,ClCH,CI + HCI (E) 100% Conv. of HCl at 275°C and 150 kPa with 5% 105,500 Ib'hr excess C2Н4 (D) 75% Conv. At 500 °C and 3000 kPa. Water is separated from the mixture before entering Figure 6.1 reactor D.arrow_forward
- Consider the following reactions: CaSO4(s) Ca²+ + SO4²- CaF₂(s) Ca²+ + 2F- Kso=10-4.59 Kso-10-10.3 a) What is the solubility of CaSO4(s) in water at 25 °C? b) What is the solubility of CaF2(s) in water at 25 °C? c) Will either of these two solids will precipitate if [F]=104 M, [SO4²¯]=10-³ M and [Ca²+]=10-² M?arrow_forwardamorphous To = 100 °C Tm= Polystyrene (PS) [CH₂-HC t semi-crystalline (-rapidly) T₂ = 100 °C Tm = 270 °C CH₂ HC CH₂ HC CH₂ HC CH₂ HC CH₂ semi-crystalline (→slowly) T₂ = 100 °C Tm= 240 °C Q2. Categorize the polymer Nylon 6 in terms a specific mode of assembly, reaction type, and mechanistic process. Provide a retrosynthetic analysis of the polymer and answer the following- c) Derive a monomer that could be employed to make Nylon 6 d) List the characteristic features of reaction type you mentioned above e) In theory, the polymer can be produced by either condensation, step- growth polymerization or by addition, chain-growth polymerization. Draw a generalized mechanistic process for step-growth synthesis of Nylon 6.arrow_forwardCalculate the solubility of Zn(OH)2 in a 0.0300M K2SO4 solution using activities and concentration. Determine the % error when using only concentrations (assuming that the actual solubility is the determined with the activity). The Kps of Zn(OH)2 is 1.2 x 10-17arrow_forward
- 1- Calculate the sp of the salt (AICI,) if the concentration of Al* is 0.022M. AICI, Alt + 3Ch 2- what is the unit of the sp of the solubility of the salt AICI.arrow_forward2. . Ethylene oxide (C₂H4O) is a high-volume chemical intermediate that is used in the manufacture of textiles, detergents, polyurethane foam, antifreeze, solvents, medicinal, adhesives, and other products like glycol and polyethylene glycol. Ethylene oxide is produced by the partial oxidation of ethylene (C₂H4) using a solid catalyst in a fixed-bed reactor: C₂H4 + O2 →→ C₂H4O An undesired reaction occurs where a portion of the ethylene reacts completely to form CO2 and H₂O: C₂H4 + O2- → CO₂ + H₂O The product gas leaving a fixed-bed ethylene oxide reactor has the following water-free composition: 22.5% C₂H4O; 70.7% N₂; 2.5% O₂; and 4.3% CO₂. Using molecular species balance, determine: a. the percent excess air based on the desired reaction b. the kg/h of the ethylene feed required to produce 75,000 MT/year of ethylene oxide c. the percent conversion of ethylene based on the desired reactionarrow_forwardCan you break down the chemical pathway for the hydrolysis of nitrile gloves (butadiene and acrylonitrile) in the environment?arrow_forward
- Examine the reaction equation below. "Heat" being present on the reactant side of an equation indicates what? "Heat" + 2NH3 N2 + 3H2arrow_forwardSolar radiation is incident on the glass cover of a solar collector at a rate of 700W/m². The glass of collector absorbs 88 percent of the incident radiation on it and has an emissivity of 0.9. The entire hot water needs of a family can be provided by a single collector of 1.2m height and 2m width. The temperature of the glass cover of the collector is measured to be 35C° on a day when the surrounding air temperature is 25C° and the wind is blowing at 30km/h. The effective sky temperature for radiation exchange between the glass cover and the open sky is -40C°. Water enters the tubes which attached to the absorber plate at a rate of 1kg/min. Assuming the back surface of the absorber plate to be heavily insulated and the only heat loss to occur through the glass cover, determine (a) the total rate of heat loss from the collector, (b) the collector efficiency, which is the ratio of the amount of heat transferred to the water to the solar energy incident on the collector, and (c) the…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The