
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
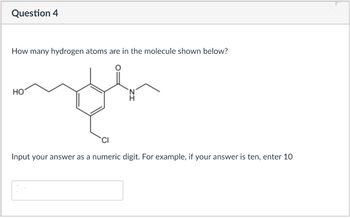
Transcribed Image Text:Question 4
How many hydrogen atoms are in the molecule shown below?
HO
Input your answer as a numeric digit. For example, if your answer is ten, enter 10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fill in the tables with the missing informationarrow_forwardClick in the answer bex to activate the palette. Determine the ompirical formula of a compound containing 2.96 g of silicon and 14.9 g of chlorine.arrow_forwardIn the blank, type your answer in number form (not words). In the following list, there are Blank 1 compounds: H₂O, PO, PO, CO, CO, CO₂, N₂O, Cs, CS2arrow_forward
- Please do this questionarrow_forwardQuestion 12 Which of these are compounds (not elements)? O NaCl O CaCI2 CaCl2 T H2 ON2arrow_forwardThis is an impossible formula. If it were real, determine the molar mass of: Pt₁ C401 Pt 195.08 g/mol = C = 12.01 g/mol O = 16.00 g/mol Make sure your answer includes TWO decimal places. Your Answer: Answer unitsarrow_forward
- Please help me answer thisarrow_forwardThe following data was collected. If it is known that compound 1 has the formula XY, Determine the formula for compound 2, then add the subscripts together to get your answer. Example: if the formula for compound 2 was XY then you would add 1+8 and your answer would be 9. Compound Mass of XMass of Y 5.12 21.504 3.42 4.77 Answer 12arrow_forwardHf728ca7011b52e183d2247#10001 Part A 15. ΑΣΦ The physician has ordered 1.0 g of tetracycline to be given every six hours to a patient. If your stock on hand is 500-mg tablets, how many will you need for one day's treatment? Express the number of tablets as an integer. Submit ? Previous Answers Request Answer 12 of 15 tablets Review | Constants | Periodic Tabarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY