
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please answer the following question to the fullest and be extremely sure that it is correct and double check. Thank you
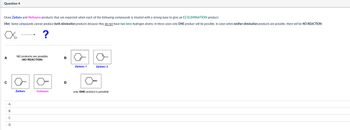
Transcribed Image Text:Question 4
Draw Zaitsev and Hofmann products that are expected when each of the following compounds is treated with a strong base to give an E2 ELIMINATION product.
Hint: Some compounds cannot produce both elimination products because they do not have two beta-hydrogen atoms: in these cases only ONE product will be possible. In cases when neither elimination products are possible, there will be NO REACTION.
?
NO products are possible
A
B
(NO REACTION)
A
B
C
D
Zaitsev 1
Zaitsev 2
Zaitsev
Hofmann
only ONE product is possible
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the concentration of unknown red dye solutionarrow_forwardBased on the data, calculate the concentration of analyte in the ORIGINAL sample in ppm. Report to 1 decimal place Sample: A 10.00 mL aliquot of the unknown was placed in a 100.0 mL volumetric flask and made up to the mark will distilled water. The absorbance of this solution is 0.185. Sample + Spike: A 10.00 mL aliquot of the unknown and a 13.00 mL aliquot of the 46.00 ppm stock were both placed in the same 100.0 mL volumetric flask and diluted to the mark with water. The absorbance of this solution is 0.326. ( Please type answer note write by hend)arrow_forwardThe concentration of hydronium ions and hydroxide ions can vary widely in a solution, easily from 1 M to 1 x 10-14 M. This variation is too wide to fit on an analog readout. In order to make this into a smaller scale suitable for an analog readout, the log to the base 10 was used so that the read out is focused on powers of 10. Note that the log [1] = 0, and the log of 1 x 10-14 = -14. It was then decided that the numbers should be positive so the sign was changed. The way to measure concentrations of both H+ and OH- was thus defined as -log. This was then referred to as p. Thus, pH = -log[H+], pOH = -log[OH-], and pK = -log[Keq]. You need to figure out how to do this mathematical manipulation on your calculator. The acid-base reaction in pure water is H2O + H2O ⇔ H3O+1 + OH-1 and it was found that in pure water under standard conditions the [H3O+1] = [OH-1] = 1 x 10-7 M Neutral pH is defined as having a [H+] or [H3O+1] of 1 x 10-7 M. The pH of a neutral solution is thus pH = -log[1 x…arrow_forward
- Calculate the concentration of unknown red dye solutionarrow_forwardAcid strength decreases in the series HI>HSO4->HF>HCN. Which of the following anions is the strongest base? a. I- b.SO42- c.F- d.CN- e. All the samearrow_forwardConcentration Fe(SCN)2+ (M) Absorbance 0.0001 0.386 0.0002 0.774 0.0003 1.154 0.0004 1.527 0.0005 1.931 Question 2 Analysis Section 1 1. Open a spreadsheet program, like Excel or Desmos, and plot your data points, with Absorbance on the y-axis and Concentration on the x-axis. 2. Fit a linear trendline and make sure the equation is labeled on your graph, including the R² value. 3. Fill in your equation below. Absorbance = -Concentration + The slope of your equation gives you the s-1 in the Beer's law equation: A = scl. Since I = 1 cm, the slope is equal to the ɛ of Fe(SCN)2+. The units of & are 1/(M*cm).arrow_forward
- 11 IMPORTANT! Please adhere to the following conditions while completing this question: 1. Use only HIGH SCHOOL knowledge to complete the question. No University level workarounds. This is so that I can understand the response. 2. Respond using TYPED WORDS, please. I know this will be harder but you are allowed to use only tools and etc... 3. Keep your response organized. This is also important as your work will likely end up in my notes.arrow_forwardI know you don't answer graded questions and I am not looking for you to straight up give me the answer to this but how do I even start? like I said before I know that the highest peak is 16amu which is Oxygen but do I just the vertical bar as a ratio? Like the next bar to the left (15amu?) is like 90% intensity? so like 9:10 ratio? There isn't even a 15amu element so please just give me some idea where to start. The place in the book it tells you to go to read has nothing about this stuff, its about finding empirical formulas from mass or percentages.... please help me!arrow_forwardA student weighed out 0.150 g of protein powder and dissolved it in 100 mL of water (Solution 1). The student then diluted this solution by transferring 1 mL into a 25 mL flask and diluting with water (Solution 2). Finally, 1 mL of that solution was transferred to a test tube and combined with 4 mL Bradford reagent. The absorbance of the solution in the test tube was 0.187. Assuming that the best fit linear line of the standard curve was y = 0.04144 x + 0.01521 (μ g mL), calculate the percent protein by mass in the original protein powder.arrow_forward
- Sel Absorbance S Verify your molar absorptivity value. Did you report your data to the correct number of significant figures? Analytical wavelength Analytical wavelength for blue dye (nm) Calibration curve Solution 1 2 3 4 5 Dye concentration (μM) 15.20 7.51 Unknown solution 3.74 1.89 1.01 Molar absorptivity (μM ¹cm-¹) Absorbance Concentration of unknown blue dye (µM) 629 Absorbance 0.756 0.432 0.259 0.175 0.144 0.083 How to calculate concentration from absorbance S A d tra 01arrow_forwardA student weighed out 0.150 g of protein powder and dissolved it in 100 mL of water (Solution 1). The student then diluted this solution by transferring 1 mL into a 25 mL flask and diluting with water (Solution 2). Finally, 1 mL of that solution was transferred to a test tube and combined with 4 mL Bradford reagent. The absorbance of the solution in the test tube was 0.11. Assuming that the best fit linear line of the standard curve was y = 0.04144 x + 0.01521 (μ g mL), calculate the percent protein by mass in the original protein powder.arrow_forwardLab Exam 1- Requires Respon X 021 Summary - CHM151-N803: Ger X A ALEKS - Delana Bailey - Learn X C Sign In or Sign Up | Chegg.com X www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgg7xIASweFMYphv4tCLy6BMpSgONHO-bYrHeBZ4S54EqYg5AJLeyt82s1i-_fz32exQQdKy96vMC... ☆ → C tab caps lock shift O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonium phosphate ((NH4),PO4) What mass of ammonium phosphate is produced by the reaction of 7.4 g of ammonia? Round your answer to 2 significant digits. esc Explanation ! 1 Q e A 1 control option @ 2 N Check W S #3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H₂PO4) with ammonia (NH₂). X H command E D X $ 4 C R FL do 5 % V T MacBook Pro 6 Y & 7 G H B Ⓒ2022 McGraw Hill LLC. All Rights Reserved. U 8 J | ΤΝΤ Μ N Nitrogen N2 gas and hydrogen X + ( 9 K 0 ) 0 Terms of Use | Privacy Center | Accessibility L 0/5 P , | 1 command option WE { [ + 11 Delana 2 | 000 Ar KI } ? D Update…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY