
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
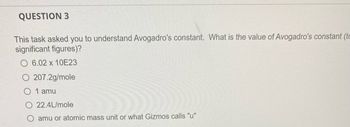
Transcribed Image Text:QUESTION 3
This task asked you to understand Avogadro's constant. What is the value of Avogadro's constant (tc
significant figures)?
6.02 x 10E23
207.2g/mole
0 1 amu
22.4L/mole
amu or atomic mass unit or what Gizmos calls "u"
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Find the percentage by mass of oxygen (O) in N2O4 if it is 30.4% nitrogen (N) by mass. %arrow_forwardO CHEMICAL REACTIONS Finding chemical formulae from a mole ratio An unknown compound has the following chemical formula: P,S where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 9.1 mol of sulfur and 3.65 mol of phosphorus. Write the complete chemical formula for the unknown compound.arrow_forwardHow many moles of oxygen are there in one mole of C6H1206? 6.02 x 1023 moles 8.00 moles 6.00 moles 2.00 moles O 1.00 molearrow_forward
- Please answer the question provided with clarityarrow_forwardHow could you calculate the Avogadro’s number [NA] for an organic compound if you know the following information: a. The volume of the molecule was calculated to be 0.664 x 10-21 cm3. b. The molar mass of the compound is 320.0 g/mole and the density is 0.8 g/ml.arrow_forwardGeneral Chemistry 4th Edition University Science Books presented by Macmillan Learning McQuarrie • Rock • Gallogly A hydrated form of copper sulfate (CUSO, · xH, 0) is heated to drive off all of the water. If there is initially 9.94 g of hydrated salt and there is 6.35 g of anhydrous CUSO, after heating, find the number of water molecules associated with each CUSO, formula unit. X =arrow_forward
- Find the number of formula units of CaCI 2 in a 1.5 kg pail of calcium chloride (used to melt Sidewalk ice). [ans: 8.1 X 10 24 formula units]arrow_forward4. The mass of silicon atoms is no. of Si atoms = 1.63x102 atom [Avogadro's O 1.04 x 10 g O 4.58 x 1022g O 2.71 x 10-25g O 7.60 x10-²g O 28.08 garrow_forwardWhat is the mass in grams of NO that can be formed from 3.18 moles of NO₂ in the reaction below? 3 NO₂ (g) + H₂O (g) → 2 HNO₃ (g) + NO (g). Round to the right amount of sig figs.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY