
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
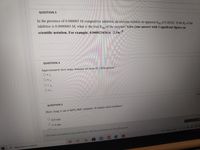
Transcribed Image Text:QUESTION 3
In the presence of 0.000005 M competitive inhibitor, an enzyme exhibits an apparent Km of 0.002M. If the Kt of the
inhibitor is 0.0000003 M, what is the true Km of the enzyme? Give your answer with 3 significant figures an
scientific notation. For example, 0.000023434 is 2.34e
QUESTION 4
Approximately how many domains are in an 82.5 kDa protein?
O a. 2
O b.3
O C. 4
O d. 1
2.5
QUESTION 5
How long is an a-helix that contains 36 amino acid residues?
6.0 nm
5.4 nm
78 Mostly clody a n
Click Save and Submit to save and submit. Click Save All Answers to save all ansuers
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A particular reaction has a ΔG‡ of 37.0 kJ mol-1. In the presence of an enzyme, the same reaction has a ΔG‡ of 5.70 kJ mol-1. Calculate the value of ΔΔG‡ in kJ mol-1.arrow_forwardIf the enzyme lactase has a Vo of 0.40 mM per minute when [S] = 1.25 mM, and a Vo of 1.0 mM per minute when [S] = 5.0 mM, what is its Vmax? Calculate the Km of the above enzyme. Calculate the slope on a Lineweaver-Burk plot (Km / Vmax) for the above enzyme.arrow_forwardDescribe how you would make the following solution containing two enzymes. 1 mL total volume in Buffer X with 10nM of enzyme A and 50nM of enzyme B You are given a solution of enzyme A at 1 mg/mL and a solution of enzyme B at 1 mg/mL. The molecular weight of enzyme A is 75,000 g/mol and the molecular weight of enzyme B is 130,000 g/mol.arrow_forward
- I need the answer as soon as possiblearrow_forwardYou run an experiment using 0.5 µM of an enzyme. The kinetic parameters for your enzyme are kcat = 1800 s-1 and KM = 25 µM. (a) If k-1 is 240 s -1, what is the value of k1? (Report your answer in units of M-1 s -1) (b) What is Vmax for the enzyme (Don't forget to include units in your answer)? (c) You set up an experiment with the following initial concentrations of substrate. Calculate the rate of the reaction at each substrate concentration (report your answer in units of µM sec-1 [S](μM) 1 100 1000 [S][μM) 1 100 1000 v (μMsec ¹) (d) You now add 10 nM of a competitive inhibitor with a KI = 2 nM. What is the rate of the reaction (v) for the following substrate concentrations and how does it compare to the rate without inhibitor (VO)? Vinhib (uMsec-¹) Vinhih/Varrow_forwardYou have an initial solution in which you added quantities of “A” and “B” such that there is 4.5 M “A” and 2.5 M “B” and no complex (“AB”) at time 0. After equilibrium, you are able to isolate and quantitate the “AB” complex, and find its concentration is 1.5 M. Given that RT is 0.59 kcal/mol, what is the delta Go’ for the association reaction?arrow_forward
- The following data were collected in the study of a new enzyme and an inhibitor of the new enzyme: Vo (nmol/sec) [S] (uM) 1.3 - Inhibitor + Inhibitor 2.50 0.62 2.6 4.00 1.42 6.5 6.30 2.65 13.0 7.60 3.12 26.0 9.00 3.58 What is the Km of the inhibited enzyme reaction?arrow_forwardyou are trying to come up with a drug to inhibit the activity of an enzyme thuogth to have a role in a liver disease. in the lab the enzyme was shown to have a Km of 1x10-6 M and Vmax of 0.1 micromoles/min.mg measruing at room temperature. you developed a mixed non-competitve inhibitor with a ki=0.4x10-6M and a Ki` pf 0/2 x 10-5 What will be the apparent Km in the presence of 1.0x10-6 M?arrow_forwardHow can you find Kcat if you are only given Vmax, Km and [E] ? I tried using Kcat=Vmax/[E] but that didn't work. How do you know if [E] is the same as [E]total, and if it isn't how do you find it from this information: The Vmax for a particular enzyme is 10 nmols/L/s. The Km for its substrate is 5 microM. If the enzyme concentration is 10 nM, what is the kcat? a.80 nmoles/L/s b.8000 nmoles/L/s c.2 nmoles/L/s d.50 nmoles/L/sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON