
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
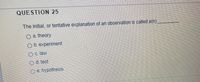
Transcribed Image Text:QUESTION 25
The initial, or tentative explanation of an observation is called a(n)
O a. theory
b. experiment
c. law
d. test
e. hypothesis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To the best of your knowledge, classify each of the following as an element, A compound, or a mixture. If it is a mixture, classify it as homogeneous or heterogeneous. a. Silver coin b. Air c.coffee d.soilarrow_forwardThe density of sulfuric acid is 1.84 g/mL. What volume of this acid will weigh 15.7 g? A. 16.0 mL B. 1.84 mL C. 28.9 mL D. 8.53 mLarrow_forwardClassify the following as a law, an experiment, or a theory. "Neon is an inert (or nonreactive) gas because it has a full electron energy shell." Group of answer choices law experiment theoryarrow_forward
- Which of the following is a physical property of matter? A. Solubility B. specific gravity C. density D. all of thesearrow_forward28. Given a problem to solve, the following steps would be following the scientific method... 1) Make an observation 2) Formulate an hypothesis 3) Perform an experiment to test your hypothesis 4) Formulate a theory that has been demonstrated by your experimentation to be true. Group of answer choices a) True b) Falsearrow_forwardWhich of the follow can NOT be possible error that occurs during a lab experiment? A. human error B. pure reactants were used C. unexpected products were produced D. the reaction didn't go to completionarrow_forward
- Intensive property is a property that does not depend on the amount of a substance. Which of the choices below is NOT an intensive property? C. hardness of metals D. heaviness of a golf ball B. yellowish coloration of urine A. the sweetness of candiesarrow_forwardWhen discussing the physical properties of solids and liquids, which one of the following is not true? Group of answer choices A.The motion of the particles increases with an increase in temperature. B.Liquids and solids are composed of small particles. C.The basic particles have significant attractions for each other. D.The basic particles are far apart.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY