
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
A revision
Transcribed Image Text:e
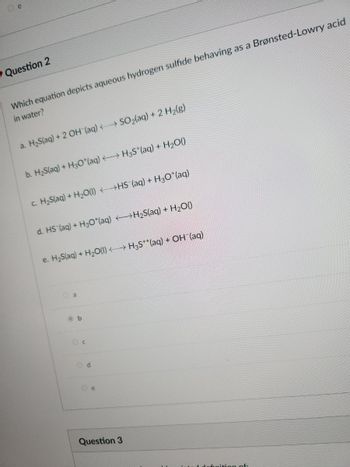
Question 2
Which equation depicts aqueous hydrogen sulfide behaving as a Brønsted-Lowry acid
in water?
a. H₂S(aq) + 2 OH(aq) →→→ SO₂(aq) + 2 H₂(g)
b. H₂S(aq) + H3O*(aq) →→→ H3S*(aq) + H₂O()
c. H₂S(aq) + H₂O(l) <HS (aq) + H₂O*(aq)
d. HS (aq) + H3O*(aq) →→→→→→H₂S(aq) + H₂O()
e. H₂S(aq) + H₂O(l) →→→ H3S**(aq) + OH (aq)
b
Od
e
Question 3
finition of:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- During the experiment, can the crucible and lid be touched with bare hands? (Choose all correct answers) No. If the crucible is still hot, a student can burn his or her hand. Yes. Gloves can be slippery, making it more dangerous to wear them in lab. Yes. They are easier to transport to the balance room and bare hands do not introduce error into the experiment. No. Dirt and oils on hands can add to the mass of the crucible.arrow_forwardAutoSave w | homework – Saved to my Mac OFF ... Home Insert Draw Design Layout References Mailings Review View O Tell me R Share O Comments Calibri (Bo. v 11 v A A E vE v E v E E Aa v AaBbCcDdEe AaBbCcD AaBbCcDdE AaBb AaBbCcDdEe Paste BIU V ab x, x A v I v A v No Spacing Normal Heading 1 Heading 2 Title Styles Pane Dictate Sensitivity Please answer the following questions fully and to the best of your ability 1) Please state how you would synthesize the following polymer below and show the mechanism behind the reaction. Note that this also means you need to tell me which molecule you are starting with as well as specifying all conditions required. он OH 2) Will either the polymer or the monomers you used to make it show peaks if IR spectroscopy analysis was done on them, and if so where? How about if UV-vis spectroscopy was performed instead? Page 1 of 1 93 words E English (United States) O Focus 白arrow_forwardгрчно чуд ч зыри: 7'чні | |Iarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY