
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
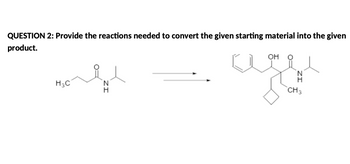
Transcribed Image Text:## QUESTION 2:
**Objective:** Provide the reactions needed to convert the given starting material into the given product.
**Starting Material:**
- Structural formula indicates an amide with a propanone group:
- Amide group (NH)
- Keto group (C=O), adjacent to a methyl group (CH₃) and an ethyl group (H₃C).
**Reaction Arrow:**
- Indicates the transformation from starting material to product.
**Product:**
- Contains:
- A cyclohexane ring
- Hydroxyl group (OH)
- Amide group similar to the starting material
- Additional hydrocarbon chains
**Explanation:**
This question involves designing a synthetic pathway for converting an amide compound to a more complex structure featuring additional substituents, such as a cyclohexane ring and a hydroxyl group. The exact reactions needed would depend on various factors such as the reactivity of functional groups and the chemistry involved.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH₂ H₂ Pt H₂ two products Lindlar's Catalyst H₂SO4, H₂O HgSOA two productsarrow_forward1) identify the reactant/s in the chemical equation and circle it, also name its major functional group 2) identify the product/s in the chemical equation (circle it) and name its functional group 3) is the a reversible reaction or not? How do we knowarrow_forward2) What is the product of the following reaction? HBr Br₂ H₂O 3) Write the product of the following reaction. CH₂OHarrow_forward
- Determine the product(s) for the chemical reaction.arrow_forwardConvert the alcohol, n-propanol, to n-propyl fluoride in 2 steps. List the reagents in the order you would use them.arrow_forwardWhich statement is true regarding the reaction shown? H₂C. CH3 H₂C (CH3)2NH H+ 2 O 1 is the final product of the reaction O 2 is the minor product and 3 is the major product 2 and 3 are formed in equal amounts O 2 is the major product and 3 is the minor product CH3 ? H3C. N 3 CH3arrow_forward
- 8. Write a balanced reaction equation, using molecular formulas, for the complete combustion of 2,2,4-trimethylpentane - a component of gasoline that has an octane rating of 100. Answerarrow_forwardWhat is the major product of this reaction? Br CH3 (CH3)3CO (CH3),CO O-Bu CH3 OI-Bu CH3 CH CH III IV %3Darrow_forwardWhat is the major product of the following reaction?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY