
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
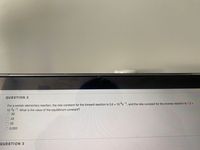
Transcribed Image Text:**Question 2**
For a certain elementary reaction, the rate constant for the forward reaction is \(3.6 \times 10^{4} \, \text{s}^{-1}\), and the rate constant for the reverse reaction is \(1.2 \times 10^{3} \, \text{s}^{-1}\). What is the value of the equilibrium constant?
- A) 30
- B) 43
- C) 23
- D) 0.033
*Note: This question does not include any graphs or diagrams.*
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Nickel and carbon monoxide react to form nickel carbonyl, like this: Ni(s)+4CO(g) →Ni(CO) 4(9) At a certain temperature, a chemist finds that a 7.4 L reaction vessel containing a mixture of nickel, carbon monoxide, and nickel carbonyl at equilibrium has the following composition: compound amount Ni 29.9 g CO 1.35 g Ni(CO) 4 0.814 g Calculate the value of the equilibrium constant ✓ for this reaction. Round your answer to 2 significant digits. K = 0 C x10arrow_forward00 For each event, indicate how the concentration of each species in the chemical equation will change to reestablish equilibrium after reactant or product is added. An up arrow indicates an increase in concentration, a down arrow indicates a decrease in concentration, and leaving it blank means there is no change in the concentration. 2 CO(g) + 0,(g) 2 CO,(g) Increasing the concentration of CO Increasing the concentration of CO2 Decreasing the volume of the container Next page age EN-MCNEIL PUBLISHING. ALL RIGHTS RESERVED. | PRIVACY POLICY | TERMS OF USEarrow_forwardNickel and carbon monoxide react to form nickel carbonyl, like this: Ni(s)+4 CO(9)→Ni(CO),(9) At a certain temperature, a chemist finds that a 4.7 L reaction vessel containing a mixture of nickel, carbon monoxide, and nickel carbonyl at equilibrium has the following composition: compound amount Ni 79.1 g CO 1.55 g Ni(CO)4 0.547 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K = 0 x10arrow_forward
- 2Please help show work and use significant figuresarrow_forwardE) reactants Reaction coordinate Problem Solving (Show your work): 23) What is the symbolic expression for the equilibrium constant for the reaction: 2 CH6(g) + 7 02(g) 4 CO2(9) +6 H20(g)? 24) What is the gas phase chemical reaction that corresponds to the equilibrium constant ex shown below? [ATTBFarrow_forwardplease answer fast!arrow_forward
- Which of the following expressions is the correct equilibrium-constant expression for the equilibrium between dinitrogen tetroxide and nitrogen dioxide? N204 (g) 2NO2 (g) INO21 A) IN2041 INO22 IN2041 B) INO21 IN204P O D) [NO2][N204] E) [NO2]2[N204]arrow_forwardDescribe the effect of the following on the rate of the reaction: S8(s) + 8 O2(g) → 8 SO2(g) 1) Decreasing the concentration of O2(g) will likely ______ the rate of the reaction. A) increaseB) deceaseC) not change 2) Decreasing the temperature will likely _____ the rate of the reaction. A) increaseB) deceaseC) not change 3) Using S8(s) in large chunks will result in _____ reaction rate than using S8(s) in fine powder.A) a faster B) a slowerC) the same 4) If no catalyst is used the reaction rate will be ______ than if a catalyst is used. A) faster B) slowerC) the samearrow_forwardWhich equilibrium constant reflects a reactant-favored equilibrium position? Question options: 0 -0.88 1.76 0.23arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY