
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
The given question was incorrectly answered, please identify what the errors made were. Then correctly answer and explain the given the question.

Transcribed Image Text:Question 2
Compound A, CSH10, is reacted with Br2 in H20. The product is then reacted with acetylide anion, C2H,
followed by Lindlar's catalyst. The resulting alkene is reacted with BH3 followed by NaOH and hydrogen
peroxide and finally Jones reagent to give final Compound B, C,H14O3. Using the provided spectral data, draw
the structure of B and label all provided spectral data. You may show the intermediate products for partial
credit.
NOTE: Ignore the side reaction that occurs in step 2. You don't know the reaction that would prevent it so I
didn't include it.
1. Br2/H20
2.
C7H14O3
3. Lindlar's cat.
4. BH3
5.H2O2, NaOH
6. Jones reagent
CSH10
Compound A
Compound B
IR (cm-1):
2745 & 1679
13CNMR (ppm, splitting):
132, s; 119, d; 26, q; 17, q; 13, q
1HNMR (ppm):
5.19, 1H, q; 1.64, 6H, s; 1.56, 3H, d
IR (cm 1):
3200 (broad), 1712 (strong)
13CNMR (ppm, DEPT):
179/none; 77/none; 42/same; 35/invert;
27/same; 8/same
HNMR (ppm):
11.8, 1H, s (exchanges w/ D2O); 4.5, 1H, s
(exchanges with D20); 2.1, 2H, d; 1.7, 1H,
sextet; 1.2, 6H, s; 0.9, 3H, d
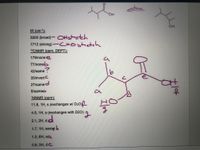
Transcribed Image Text:IR (cm-1):
3200 (broad)- OHstretch
1712 (strong)-CEogtretch
13CNMR (ppm, DEPT):
179/none e
77/nonea
42/same
35/invertC
27/same
a
to
8/sameca
1HNMR (ppm):
11.8, 1H, s (exchanges w/ D20)
4.5, 1H, s (exchanges with D20) q
p"
2.1, 2H, de
1.7, 1H, sextgt b
1.2, 6H, sC
0.9, 3H, dC
0:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] Use the References to access important values if needed for this question. Taking logarithms and antilogarithms is necessary to solve many chemistry problems. For practice, complete the following table, where N is a number. log N 7.92 1.867 -1.402 Submit Answer Retry Entire Group 4 more group attempts remaining Previous Next Save and Exitarrow_forwardExplain if it is possible to combine different types of mass analyzer.arrow_forwardRow two is incorrectarrow_forward
- If this piece of blue litmus paper is placed in that acid solution,then this piece of blue litmus paper will turn red.which of the following is true of this conditional statement: A. The antecedent is "this piece of blue litmus paper will turn red." B. This statement is false. C. This statement cannot serve as a premise in an argument. D. This statement cannot be the conclusion of an argument.arrow_forwardState the effects that cause bulk and nanoscale materials to have different magnetic properties.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY