
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
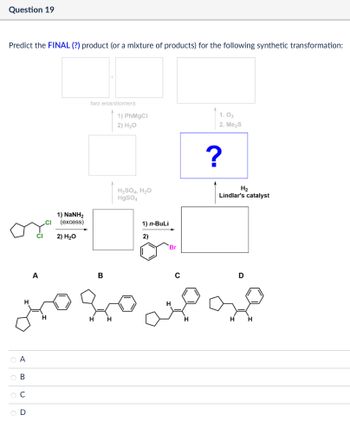
Transcribed Image Text:Question 19
Predict the FINAL (?) product (or a mixture of products) for the following synthetic transformation:
A
OA
ов
BCD
two enantiomers
1) PhMgCl
2) H₂O
1) NaNH2
(excess)
2) H₂O
H2SO4, H₂O
HgSO4
1) n-BuLi
2)
Br
16
B
1.03
2. Me₂S
?
H₂
Lindlar's catalyst
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is(are) the major final product(s) formed during the following sequence of reactions? 01 11 V 1.0₂ 2. (CH₂)₂S C₂H₂O 2 identical molecules ||| =PPhy < IV орнаarrow_forwardShort Anver Question 11. Povide ONLY the MAJOR NO MECHANISMS for the following reactions-you include the regiochemistry and stochemistry when necessary HEY ROOR ✓ 1)0, 2) DMS 1) H₂0Ac), HO 2) Na 1) R₂BH 2) NaOH, H₂O₂arrow_forwardCan you please answer questions d-j please!arrow_forward
- Which series of reactions would carry out the following transformation? OTS ? ► View Available Hint(s) NH₂ O 1. NH3 2. NaBH4 O 1. Phthalimide, KOH, H₂O 2. NH₂NH₂, heat O 1. NaN3 2. LIAIH4 3. H₂O O 1. NaCN 2. H₂, Pdarrow_forwardof 55 > $ 4 Macmillan Learning 101 LL Organic Chemistry Maxwell a) Use three curved arrows to show the elimination of the first hydrogen bromide. Select Draw Templates More presented by Macmillan Learning Complete the curved arrow mechanism of the following double elimination reaction when 2,3-dibromopentane is treated with two equivalents of sodium amide and heated in mineral oil. 15 % / ||| ||| Q Search ***** 5 G : NH₂ f6 6 C H H : Br : Y H Br N ** : Br: & 7 N lipi fg U Erase * 8 N 19 M 144 K Resources b) Use three curved arrows to show the elimination of the second hydrogen bromide. Select Draw Templates lates f10 |||||| C H : NH3 ► 11 Lx Give Up? : Br : More H P Br N : Br: ** : NH₂ F12 ** + Hint Erase BANG & OLUFSEN insert ] р barrow_forwardQuestion 20 pleasearrow_forward
- Question 27 of 30 > O Macmillan Learning Organic Chemistry Loudon | Parise SEVENTH EDITION Given the biaryl product, select the two reactants that would give this product via a Suzuki coupling. reactants Select two reactants. Pd(PPH3)4 heat, Na₂CO3 ·C=CH Br H H Br MgBr presented by Macmillan Learningarrow_forwardWhat is the organic product formed in the following sequence of reactions? Br (1) Mg/ether, (2) CO₂ HOCH₂/H* (1) DIBAL-H, toluene, -78°C heat (2) H*(aq) (3) H*(aq) II H III IV de "a H H I ?arrow_forward24. Which product is formed by the reaction ? A) | B) || C) ||| D) IV Br ОН NaH Br ... هستم IVarrow_forward
- Cambridge International AS Level Chemistry ot boe slo plgmes s al snsdismonooli End-of-chapter questions 1 1-bromobutane will undergo reactions when heated, as shown by reactions A and B. ot dwab on s bl auoltse s boaub svsd 20 swoll gab ods-suai la yel snoso sdr ssiganm n2 monl gnivims noiteibar VU luad CH;CH,CH,CH,Br B CH;CH,CH,CH,OH CH;CH,CH=CH, 90u S1s 2O1D Isdh tuo gomia sli ni qu dgid tod a guch pecoue For reactions A and B give the reagents used in each case. b Reaction A was repeated using 1-iodobutane instead of 1-bromobutane. Explain any difference in the rate of reaction observed. p bas- od mon c What type of organic reaction is A? d Show the mechanism for reaction A. e Reaction A was repeated with 2-bromo-2-methylpropane instead of 1-bromobutane. i Name the organic compound formed. elle ii The mechanism of the reaction with 2-bromo-2-methylpropane differs from the mechanism of smitas nosd asd i anol reaction A. Describe how the mechanisms differ. f What type of reaction is…arrow_forwardpe12 Part 2 ad Crapter 1 O NWP Assessment Player UI Appli x ui/v2/assessment-player/index.html?launchld3De7f9ff7e-311b-440d-8832-4aeb728ebe50#/question/25 13 WileyPLUS HW Question 26 of 30 - / 1 OH H30* HO Consider the epoxide ring opening reaction shown. Which of the following represents a step in the mechanism, with correctly drawn curved arrows? HO: H2Ö: HO: Sáve for Later Attempts: 0 of 3 used Submit AnswE DELLarrow_forwardPlease don't provide handwriting solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning