
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:At 896 K, Kp = 0.471 for the equi x +
ucumberlands.blackboard.com/ultra/courses/_153948_1/cl/outline
P
X
✓ Question Completion Status:
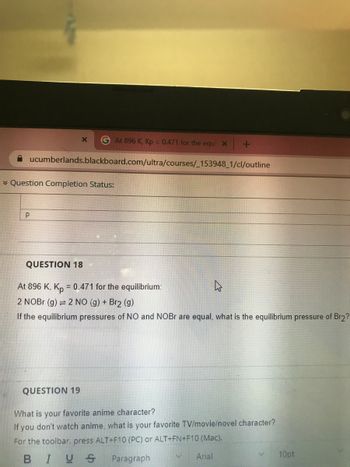
QUESTION 18
At 896 K, Kp = 0.471 for the equilibrium:
2 NOBr (g) = 2 NO (g) + Br2 (g)
If the equilibrium pressures of NO and NOBr are equal, what is the equilibrium pressure of Br2?
QUESTION 19
What is your favorite anime character?
If you don't watch anime, what is your favorite TV/movie/novel character?
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
BIUS
Paragraph
N Arial
10pt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction:2NOBr(g) 2NO(g) + Br2(g) If 0.224 moles of NOBr, 0.330 moles of NO, and 0.384 moles of Br2 are at equilibrium in a 14.0 L container at 518 K, the value of the equilibrium constant, Kp, is .arrow_forwardQ2arrow_forwardConsider the reaction. 2 A(g) = B(g) Kp = 5.62 x 10-5 at 500 K If a sample of A(g) at 4.20 atm is heated to 500 K, what is the pressure of B(g) at equilibrium? PB = atmarrow_forward
- 4. IBr decomposes according to the reaction at 150 C: 2 IBr (g) = I2 (g) + Br2 (g) If IBr is placed in a container at an initial pressure of 5.0 atm, what will be its equilibrium pressure at 150°C? K, = 8.3 x 10-3arrow_forwardThe equilibrium constant for the following reaction is 67.6 at 232 °C. PCI 3(g) + Cl₂(g) PCI5(g) K = 67.6 at 232 °C Calculate the equilibrium constant for the following reactions at 232 °C. (a) PCI5(g) PCl3(g) + Cl₂(g) K= (b) 2 PCI5(g) 2 PCI3(g) + 2 Cl₂(g) K=arrow_forwardA student ran the following reaction in the laboratory at 588 K: CO(g) + Cl₂(g) —COCI₂(g) When she introduced CO(g) and Cl₂(g) into a 1.00 L evacuated container, so that the initial partial pressure of CO was 1.74 atm and the initial partial pressure of Cl2 was 0.951 atm, she found that the equilibrium partial pressure of COCI₂ was 0.685 atm. Calculate the equilibrium constant, Kp, she obtained for this reaction. Кр =arrow_forward
- A student ran the following reaction in the laboratory at 591 K:COCl2(g) CO(g) + Cl2(g)When she introduced COCl2(g) at a pressure of 0.380 atm into a 1.00 L evacuated container, she found the equilibrium partial pressure of Cl2(g) to be 0.247 atm.Calculate the equilibrium constant, Kp, she obtained for this reaction.arrow_forwardGiven the following reaction at equilibrium, if Kc= 1.90 x 10 19 at 25.0 °C, Kp =. H2 (g) +Br2 (g) 2HBr (g) 5.26 x 10-20 1.56 x 104 6.44 x 105 1.90 x 1019 none of thesearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY