
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
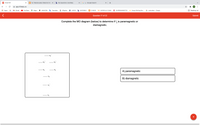
Transcribed Image Text:**Title: Molecular Orbital Theory: Understanding Magnetism in I₂**
**Task:**
Complete the MO diagram (below) to determine if I₂ is paramagnetic or diamagnetic.
**Molecular Orbital (MO) Diagram:**
The diagram consists of molecular orbitals represented by horizontal lines. Electrons are indicated by arrows filling these orbitals.
From bottom to top, the orbitals are labeled as follows:
1. **σs**: Lowest energy non-bonding orbital where electrons first populate.
2. **σs***: Anti-bonding orbital, indicated by an asterisk (*).
3. **σp**: Bonding orbital formed from the combination of p-orbitals.
4. **πp**: Two degenerate π (pi) orbitals that allow lateral overlap.
5. **πp\***: Two degenerate anti-bonding pi orbitals, indicated by an asterisk (*).
6. **σp\***: Highest energy orbital, also anti-bonding, indicated by an asterisk (*).
**Question:**
Is I₂:
A) Paramagnetic: Has unpaired electrons.
B) Diamagnetic: All electrons are paired.
**Instruction:**
Analyze the filled molecular orbitals for unpaired electrons to determine the magnetic property of I₂.
**Note:** For accurate results, fill the orbitals according to the electron configuration for I₂, considering the energy order and pauli exclusion principle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question: In the context of quantum mechanics and molecular orbital theory, how do non-bonding orbitals contribute to the stability of a molecule, and how do their electron density distributions affect the molecule's reactivity? Provide an example to illustrate your point.arrow_forwardUse a MO diagram and calculate the bond order for BO+arrow_forward(a) If these three balloons are all the same size, what angleis formed between the red one and the green one? (b) If additionalair is added to the blue balloon so that it gets larger,will the angle between the red and green balloons increase,decrease, or stay the same? (c) Which of the following aspectsof the VSEPR model is illustrated by part (b): (i) Theelectron-domain geometry for four electron domains is tetrahedral.(ii) The electron domains for nonbonding pairs arelarger than those for bonding pairs. (iii) The hybridizationthat corresponds to a trigonal planar electron-domain geometryis sp2. [Section 9.2]arrow_forward
- could you draw the diagram on paper?arrow_forwardIn reference to the following figure, which of the following statements is true? a. An energy level diagram can be made of the two overlapping 1s orbitals, and the Aufbau process used to determine the electronic configurations of H2 and He2. b. In-phase overlap of electron waves represented by the two 1s orbitals results in a new orbital having lower energy than either of the s orbitals. This new bonding orbital concentrates the electron probability between the two nuclei. c. Out-of-phase overlap between two 1s orbitals results in a new orbital having higher energy than either of the s orbitals. This new antibonding orbital would place most of the electron probability to the left and right of the two nuclei. d. The energy-lowering of the bonding molecular orbital and energy-raising of the antibonding molecular orbital with respect to the atomic orbitals will result in equal energy splitting with no net bonding between helium atoms. e. All of the above statements are true.arrow_forwardFor HCl (note that the IEH = 13.6 eV and IEC = 13.0 eV): (a) Draw a MO diagram and write the ground-state electron configuration. (You do not need to show core orbitals.) Be sure to give each molecular orbital its correct label, including non-bonding orbitals. What is the bond order?arrow_forward
- a) Draw the molecular orbital diagram of the given compounds, predict the bond order, and une comment on whether the molecule can exist or not. F₂ Be₂ b) Is CN¯diamagnetic or paramagnetic? c) Would you expect the bond in CN to be stronger or weaker than in the F₂ molecule? why? CN "arrow_forwardQ3) What are the orbital hybridization for each of the following: Explain 1- ВС 2- C,H, 3- Cr(CO), 4- Fe(CO), 5- CH, 6- PtBr,arrow_forwardDraw the MO energy diagram for FO+ 1. What is the bond order for FO+ 2. Is FO+ diamagnetic or paramagnetic, defend your answer. Thank you!arrow_forward
- Determine which diamotic substances are paramagnetic based on their molecular orbital electronic configuration a) Ne2 b) C2 c) B2 d) F2 e) N2arrow_forwardDraw a molecular orbital energy diagram for ClF.arrow_forwardThe atomic orbital, AO, contributions of penta-1,3-dienyl cation (shown to the right) are drawn in random order below. Using the A-E labels: (a) match the AO contributor to the MO energy diagram levels, (b) next to the MO diagram levels, identify each π MO as either bonding, nonbonding, or antibonding, (c) populate the T electrons in the appropriate MO energy levels, and (d) label the HOMO and the LUMO. 8 8 8 8 A (11 o* MOs) |||| (11 g Mos) B с D Earrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY