
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
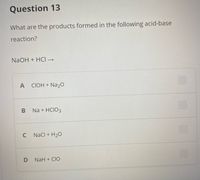
Transcribed Image Text:Question 13
What are the products formed in the following acid-base
reaction?
NaOH + HCI →
A CIOH + Na20
Na + HCIO3
C
Nacl + H20
NaH + CIO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer the following questions about acid-base equilibrium reactions (d)Based on the following Kavalues, which is the strongest acid and which is the weakest acid? Ka (HNO2) = 4.5 x 10 –4Ka (H2SO3) = 1.5 x 10 –2Ka (H2CO3) = 4.3 x 10 –7arrow_forwardComplete the reactions below, briefly explaining in each case type of reaction taking place. i. Δ N-Ph ??? 1. Br2, NaOH ii. ??? 2. H+, H₂O + CHBг3 OHarrow_forwardConsider the acid-base reaction below: (Question 14) Ca(HCO3)2 + Ca(OH)2 ----> Compound A + H2O (OR Ca(HCO3)2 + Ca left parenthesis OH right parenthesis 2 ----> Compound A + H2O) One of the single ions that is used to form compound A is ____. Assume enough of each compound is used to get a complete reaction. Question 14 answer choice options: C32- (OR C3 2 minus) C43- (OR C4 3 minus) C3- (OR C 3 minus) CO- (OR C O 1 minus) CO2- (OR C O 2 minus) CO43- (OR C O4 3 minus) CO4- (OR C O4 1 minus) C22- (OR C2 2 minus) CO32- (OR C O3 2 minus) C4- (OR C4 1 minus) C2- (OR C 2 minus) C33- (OR C3 3 minus) CO6- (OR C O 6 minus) C23- (OR C2 3 minus) C2- (OR C2 1 minus) C- (OR C 1 minus) C3- (OR C3 1 minus)…arrow_forward
- Question 17 For the following acid-base reaction, predict which side of the equilibrium is favored. C=N + N. H-C=N + „N.arrow_forwardWrite the reaction that happens with Nile Blue A and NaOH. Given is the compound, Nile Blue A. Thank youarrow_forwardWrite the balanced chemical reaction showing the neutralization of aqueous acetic acid with aqueous strontium hydroxide.arrow_forward
- Show the structures of the missing substance or substances in the following acid-base equilibriaarrow_forwardIdentify the class type of compound shown: NaCl Strong Base Strong Acid O Weak Base Neutral O Weak Acidarrow_forwardRank the following solutions according to their acidity: Rank Solution 1 pH = 11 [H3O+] = 105 M Solution 3 [OH-] = 10-8 M Solution 2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY