
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Content
box
Plato's Euthyphro - Part I X Courses
d.umbc.edu/ultra/courses/_68592_1/cl/outline
oxidation of iodite ions a X L is cbr4 pola
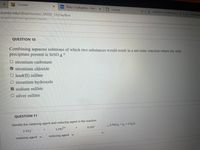
QUESTION 10
Combining aqueous solutions of which two substances would result in a net ionic reaction where the only
precipitate present is SRSO 4?
O strontium carbonate
strontium chloride
O lead(II) sulfate
O strontium hydroxide
V sodium sulfate
O silver sulfate
QUESTION 11
Identify the oxidizing agent and reducing agent in the reaction.
5 Pb2+
5 PbO2 + 12 + 4 H20
8 ОН
2 103
reducing agent v
oxidizing agent v
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 26arrow_forwardPls help ASAP. Pls help on both PLSarrow_forwardUse the precipitation interactive to answer the questions. Select the color of the solid that forms when Ni(NO3)2Ni(NO3)2 solution is added to K2SK2S solution. white green black yellow Complete the net ionic equation for the reaction. Cation ++ Anion ⟶⟶ Precipitatearrow_forward
- Aqueous solutions of 0.1 M copper (II) chloride and 0.1 M silver nitrate are combined and allowed to react. From the following, select all of the statements that are true. A The product of the reaction will have a colored supernatant B The product of the reaction will have a colorless supernatant C The reaction will produce a gas D The reaction will produce a precipitate E The reaction will produce water as a product F The reaction will result in an oxidation and/or reduction of one of the reactantsarrow_forwardDetermine the spectator ions for the following reaction: A solution of Mgl2 reacts with a solution of AgNO3. O None of these choices are correct. O Mg2+ O Ag* O 02- NO3 O NS+arrow_forwardFirst, assign oxidation numbers for each atom in the balanced chemical equation below. You'll find it easiest to fill the blanks in left to right since once there is an oxidation number typed in the blank it will be much closer to appearing over the appropriate elemental symbol. Blank 1 Blank 2Blank 3 12Blank 13 Blank 4Blank 5Blank 6 Blank 14Blank 15Blank 16 KMnO4(aq) + KCIO₂(aq) + H₂O(l) → 4 MnO₂(s) + 3 KCIO4(aq) + 4 KOH(aq) Now, identify each of the following What underwent reduction? Blank 17 Blank 7Blank 8 What gained electrons? Blank 18 • What got oxidized? Blank 19 What is the oxidizing agent? Blank 20 • What is the reducer? Blank 21 What lost electrons? Blank 22 Blank 9Blank 10 Blank 11 Blankarrow_forward
- What element, present among the reactants, is reduced in the following ration?arrow_forwardClassify Pb (s) + 2 AGNO3 (aq) decomposition, single-displacement, or double-displacement reaction: Pb(NO3)2 (aq) + 2 Ag (6) as a synthesis, double-displacement decomposition single-displacement synthesisarrow_forwardThis reaction represents the titration from today's lab. 2- 2 S₂03² + 1₂ → S406² +21 O In this reaction, [Select] ✓ [Select] S406 1- 12 S203 is the oxidizing agent and is the reducing agent.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY