
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
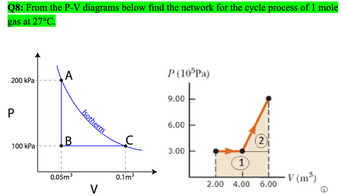
Transcribed Image Text:Q8: From the P-V diagrams below find the network for the cycle process of 1 mole
gas at 27°C.
A
P (105Pa)
200 kPa
P
B
100 kPa
0.05m³
Isotherm
0.1m³
V
9.00
6.00
3.00
2
1
V (m³)
2.00 4.00
6.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- A 1.00-mol sample of an ideal gas ( = 1.40) is carried through the Carnot cycle described in Figure 22.11. At point A, the pressure is 25.0 atm and the temperature is 600 K. At point C, the pressure is 1.00 atm and the temperature is 400 K. (a) Determine the pressures and volumes at points A, B, C, and D. (b) Calculate the net work done per cycle.arrow_forwardUse a PV diagram such as the one in Figure 22.2 (page 653) to figure out how you could modify an engine to increase the work done.arrow_forwardA sample of a monatomic ideal gas is contained in a cylinder with a piston. Its stale is represented by the dot in the PV diagram shown in Figure OQ22.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality.arrow_forward
- A heliumfilled toy balloon has a gauge pressure of 0.200 atm and a volume of 10.0 L. How much greater is the internal energy of the helium in the balloon than it would be at zero gauge pressure?arrow_forwardDoes a gas become more orderly when it liquefies? Does its entropy change? If s, does the entropy increase or decrease? Explain your answer.arrow_forwardIt is unlikely that a process can be isothermal unless it is a very slow process. Explain why. Is the same true for isobaric and isochoric processes? Explain your answer.arrow_forward
- (a) Construct a table showing the macro states and all of the individual microstates for tossing 13 coins. (Use Table 15.5 as a guide.) (b) How many macro states are there? (c) What is the total number of microstates? (d) What percent chance is here of tossing 5 heads and 1 tail? (e) How much more likely are you to toss 3 heads and 3 tails than 5 heads and 1 tail? (Take me ratio of the number of microstates to find out.)arrow_forwardYou have a particular interest in automobile engines, so you have secured a co-op position at an automobile company while you attend school. Your supervisor is helping you to learn about the operation of an internal combustion engine. She gives you the following assignment, related to a simulation of a new engine she is designing. A gas, beginning at PA = 1.00 atm, VA = 0.500 L, and TA = 27.0C, is compressed from point A on the PV diagram in Figure P19.31 (page 530) to point B. This represents the compression stroke in a fourcycle gasoline engine. At that point, 132 J of energy is delivered to the gas at constant volume, taking the gas to point C. This represents the transformation of potential energy in the gasoline to internal energy when the spark plug fires. Your supervisor tells you that the internal energy of a gas is proportional to temperature (as we shall find in Chapter 20), the internal energy of the gas at point A is 200 J, and she wants to know what the temperature of the gas is at point C. Figure P19.31arrow_forwardUnreasonable Results (a) How many moles per cubic meter of an ideal gas are there at a pressure of 1.001014N/m2 and at 0C ? (b) What is unreasonable about this result? (c) Which premise or assumption is responsible?arrow_forward
- A 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? Find (c) Q, (d) Eint, and (e) W for the gas during this process.arrow_forward(a) What is the change in entropy if you start with 10 coins in the 5 heads and 5 tails macrostate, toss them, and get 2 heads and 8 tails? (b) How much more likely is 5 heads and 5 tails than 2 heads and 8 tails? (Take the ratio of the number of microstates to find out.) (c) If you were betting on 2 heads and 8 tails would you accept odds of 252 to 45? Explain Why or why not. Table 15.5 10Coin Toss MacrostateNumber of Microstates (W) Heads Tails 10 0 1 9 1 10 8 2 45 7 3 120 6 4 210 5 5 252 4 6 210 3 7 120 2 8 45 1 9 10 0 10 1 Total: 1024arrow_forwardAssume a sample of an ideal gas is at room temperature. What action will necessarily make the entropy of the sample increase? (a) Transfer energy into it by heat, (b) Transfer energy into it irreversibly by heat, (c) Do work on it. (d) Increase either its temperature or its volume, without letting the other variable decrease, (e) None of those choices is correct.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning