
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
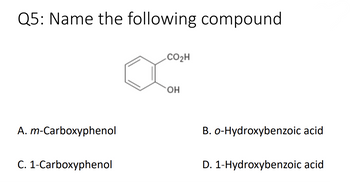
Transcribed Image Text:Q5: Name the following compound
A. m-Carboxyphenol
C. 1-Carboxyphenol
CO₂H
OH
B. o-Hydroxybenzoic acid
D. 1-Hydroxybenzoic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Acid anhydride Carboxylic Acid H₂C CH₂OH H₂O CH,OH CH3 Ester H₂C O CH₂OH 1. CH₂MgBr 2. H₂O* Acid chloride CH,CH,NH, Tertiary alcohol Amidearrow_forwardCH₂-CH₂-CH- -0-CH₂ 1 CH₂ Enter the name of the carboxylic acid first, then the name of the alcohol. Separate the names with a comma.arrow_forwardwhat are the products of the reaction of ethylene oxide and aluminum trichloridearrow_forward
- One choose the most appropriate name for the following organic molecule. Hexanone, heptanoic acid, hexanoic acid, hexanol, heptanone, or hexanal.arrow_forwardIdentify the organic functional groups and reaction type for the following reaction.The reactant is a(n)a. secondary amideb. carboxylic acidc. tertiary amided. aromatice. ketonef. primary amideg. aldehydeh. amineThe products are a(n)a. carboxylate ion and amineb. ketone and aminec. carboxylic acid and amided. carboxylic acid and alcohole. ester and aminef. aldehyde and amineg. carboxylic acid and ammonium ionThe reaction type isa. hydrolysis (in acid)b. amide synthesisc. hydrationd. esterificatione. dehydrationf. hydrolysis (in base)arrow_forwardBoth A) C=0 group and E) -C(=O) NH2 group is incorrect. Please help me.arrow_forward
- A. Identify the name of the functional group where the given organic compounds belong (alcohol, ether, aldehyde, ketone, carboxylic acid, ester, amine) 1. H H Br H H -C C-H H H H 2. H H Н—с H -5- H H N- H- 3. H H -нн I-arrow_forwardCream of tartar is a white powder sometimes used in baking. (It's what separates a tangy, chewy snickerdoodle from an ordinary cinnamon-coated sugar cookie. The acid in the cream of tartar gives snickerdoodles their distinctive tangy flavor, and the chew happens because cream of tartar prevents sugar in the cookie dough from crystalizing into crunchiness. Allrecipies.com) Cream of tartar (KHC4H4O6) is the conjugate base salt of tartaric acid (shown to the right). The Ka for another similar acid is 8.7×10-3. What is the standard Gibb's free energy (in kJ/mol) for the dissociation of that other acid?arrow_forwardWhat is this compound?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning