
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
None
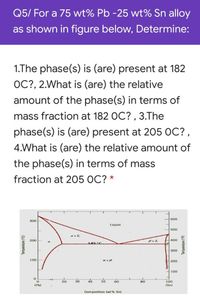
Transcribed Image Text:Q5/ For a 75 wt% Pb -25 wt% Sn alloy
as shown in figure below, Determine:
1.The phase(s) is (are) present at 182
OC?, 2.What is (are) the relative
amount of the phase(s) in terms of
mass fraction at 182 OC? , 3.The
phase(s) is (are) present at 205 OC?,
4.What is (are) the relative amount of
the phase(s) in terms of mass
fraction at 205 OC? *
600
300
Liquid
500
200
400
185°C
300
100
200
100
20
30
40
50
60
80
100
(Pb)
(Sn)
Composition (wt % Sn)
Temperature (°C)
Termperature ("F)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider the phase diagram below. The three points A, B, and C are at concentrations of 26.4, 31.3, and 34 wt% Ni respectively. The ends of the tie line are at C1 = 25% wt% Ni and C2 = 35 wt% Ni. What are the weight fractions of the alpha phase at A and the L phase at B, as well as the alpha phase/L phase ratio at C? T(°C) 1300 L (liquid) 1200 20 A B C L + a C1 S liquidus L + a solidus a (solid) 50 wt% Ni a. Walpha=0.21; WL=0.33; Walpha/WL = 13.17 b. Walpha=0.09; WL=0.32; Walpha/WL = 15.00 c. Walpha=0.07; WL=0.46; Walpha/WL = 11.17 d. Walpha=0.14; WL=0.37; Walpha/WL = 9.00arrow_forwardConsider the phase diagram below. The three points A, B, and C are at concentrations of 27, 31.9, and 33.8 wt% Ni respectively. The ends of the tie line are at C1 = 25% wt% Ni and C2 = 35 wt% Ni. What are the weight fractions of the alpha phase at A and the L phase at B, as well as the alpha phase/L phase ratio at C? T(°C) 1300-L (liquid) 1200 20 ABC L + a C1 30 S C2 liquidus 40 L + a solidus α (solid). O a. Walpha=0.12; WL=0.41; Walpha/WL = 9.41 O b. Walpha=0.27; WL-0.26; Walpha/WL = 11.11 O c. Walpha=0.15; WL-0.26; Walpha/WL = 11.80 Od. Walpha=0.20; WL-0.31; Walpha/WL = 7.33 50 wt% Niarrow_forwardPhase diagram of lead-tin system is given in the figure below. For the of composition for 80 wt.% Sn-20 wt.% Pb: (a) Draw schematic sketches of the microstructure, (b) calculate the compositions and weight fractions of each phases at 280°C, just above 183°C (T ) and just below 183°C (T). T° C) 300 L (liquid) TL+BB 97.8 200- 18.3 183° С 1 %3D 3D 100- a + B 13 20 100 32 60 80 C, wt% Snarrow_forward
- Consider the phase diagram below. The three points A, B, and C are at concentrations of 27.8, 31.6, and 33.5 wt% Ni respectively. The ends of the tie line are at C1 = 25% wt% Ni and C2 = 35 wt% Ni. What are the weight fractions of the alpha phase at A and the L phase at B, as well as the alpha phase/L phase ratio at C? T(°C) 1300 1200 - L (liquid) 20 ABC L + a C1 30 S C2 liquidus 40 L + a solidus α (solid) a. Walpha=0.34; WL=0.29; Walpha/WL = 8.94 b. Walpha=0.23; WL=0.29; Walpha/WL = 8.85 c. Walpha=0.28; WL=0.34; Walpha/WL = 5.67 d. Walpha=0.19; WL=0.44; Walpha/WL = 7.56 50 wt% Niarrow_forward5. For 40 wt% Sn – 60 wt% Pb alloy at 150 oC (at point B) as shown in Figure, ii. Calculate the composition(s) of the phase(s) present. iii. Calculate the amount of each phase present in terms of mass fraction. 600 300 Liquid 500 a+L 200 400 300 100 200 100 20 60 80 100 (Pb) (Sn) Composition (wt% Sn) Temperature ("C) Temperat ure ("F)arrow_forwardCould someone explain in detail how to solve this problem please. I really dont understand it. Thank you so much.arrow_forward
- Referring to Figure 1, please answer the following [NOTE: SHOW YOUR WORK on Figure 1]:a. provide the specific name for the phase boundary lines denoted by A and B. b. identify the phases present at equilibrium in the phase fields denoted by C, D, and E. c. identify the specific name for the point denoted by F. d. What is the maximum solid solubility of Mg in Al? At what temperature does it occur? e. An Al-Mg alloy (10 wt% Mg, 90 wt% Al) is heated slowly (to insure equilibrium) from a temperature of 200 C: i. At what temperature does the first liquid phase form? ii. What is the composition of the liquid phase at the temperature in part i.? iii. At what temperature does complete melting (no solid phase remaining) occur? iv. What is the composition of the last solid remaining prior to complete melting? f. Using the equilibrium phase diagram of Figure 1, identify the phases present, their compositions, and their relative mass fractions at equilibrium for a 70 wt% Mg – 30 wt% Al…arrow_forwardanswer quicklyarrow_forward5. For a 33 wt% Sn-67 wt% Pb alloy at 150°C: a. What phase(s) is (are) present? b. (b) What is (are) the composition(s) of the phase(s)? C. Calculate the relative amount of each phase present in terms of mass fraction. Temperature (°C) 300 200 100 327°C (Pb) 20 a + L 18.3 40 20 Composition (at % Sn) 60 183°C 40 a + ß Liquid 61.9 60 Composition (wt% Sn) Lead - Tin Phase Diagram 80 80 B+L 232°C 97.8 100 B | to 600 500 400 300 200 100 100 (Sn) Temperature (°F)arrow_forward
- Consider Cu - Ag Phase diagram below: 1200 A -Liquidus 1000 Liquid -Solidus 779°C (TE) 800 Temperature (°C) 600 400 a C 200 0 B 8.0 (Cag) Solvus a +L a + ß E 71.9 (CE) B+L 91.2 (CBE) B H 1 1 1 1 1 { ( L 20 40 60 80 100 (Cu) Composition (wt% Ag) (Ag) Consider 71.9% Ag-28.1% Cu, which is cooled below 779°C. What is the composition of the phases present just below 779°C and give the amount of beta phase presentarrow_forwardPhase Diagram A/ The lead and tin are partially dissolved in each other in solid state although they completely dissolved in liguid state. They form eutectic from solid solutions alfa (a) and beta (B) at alloying composition 62% tin and 38% lead, the eutectic forming temperature is 184oC. Alfa contained 20% tin dissolved in 80% lead at 1840C while beta consist of lead dissolved in tin at 1840C. If the 100 g of eutectic contained 45.5 g of alfa at 1840C. The melting temperatures of lead and tin are 3290C and 2320C respectively, and the solubility of tin and lead in each other's diminished to zero at room temperature. Plot the phase diagram for lead- tin system label all phases and temperature. Show graphically the temperature of the formation of the first solid for 42% lead alloyarrow_forwardIn a binary phase diagram ( pressure omitted) what is the maximum number of phases that can coexist for at least one degree of freedom?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY