
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
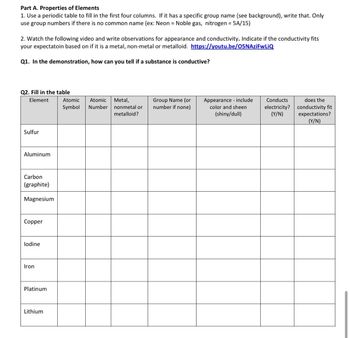
Transcribed Image Text:Part A. Properties of Elements
1. Use a periodic table to fill in the first four columns. If it has a specific group name (see background), write that. Only
use group numbers if there is no common name (ex: Neon = Noble gas, nitrogen = 5A/15)
2. Watch the following video and write observations for appearance and conductivity. Indicate if the conductivity fits
your expectatoin based on if it is a metal, non-metal or metalloid. https://youtu.be/O5NAziFwLiQ
Q1. In the demonstration, how can you tell if a substance is conductive?
Q2. Fill in the table
Element
Sulfur
Aluminum
Carbon
(graphite)
Magnesium
Copper
lodine
Iron
Platinum
Lithium
Atomic Atomic
Symbol Number
Metal,
nonmetal or
metalloid?
Group Name (or
number if none)
Appearance include
color and sheen
(shiny/dull)
Conducts
electricity?
(Y/N)
does the
conductivity fit
expectations?
(Y/N)
Expert Solution
arrow_forward
Step 1
We are given different elements(metals and nonmetals), and we have to find their atomic symbol, atomic number, identify metal or nonmetal, group name, appearance, and conductivity, and fill in the columns.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the chart please fill in the names and chemical formula for the 4th Line (Sr2+) line the whole column is 1 question thank you!!!!arrow_forwardQUESTIO Complete the table of ion names and symbols (use superscript and subscript notation buttons above: X² and X₂) 1 2 3 4 5 lon name magnesium ion 12pt phosphide ion manganese(II) ion Edit View Insert Format Tools Table | B IU A Paragraph T² GO Symbol 0²- Cr3+ DA V ...arrow_forwardWhich of the following would be considered as a rare earth? Group of answer choices cadmium cesium cerium curium Which of the following is the polyatomic ion called carbonate? Group of answer choicesarrow_forward
- Which element(s) would you expect to find in box #6? 1 919 2 0 0 0 Si 0 0 3 Si 0 4 5 60 Oxygen (O) Magnesium (Mg) Potassium (K) ☐ Sodium (Na) ☐ Calcium (Ca) ☐ none of the responses are correctarrow_forwardUsing the chart please fill in the names and chemical formula for the 5th Line (Al3++) line the whole column is 1 question thank you!!!!arrow_forwardCanvas Question 47 Consider the diisotopic element represented below. element? O forms colored compounds O boils at -186 C O melts at 29.8 C O electrically conductive Question 48 Isotope Isotopic Mass (amu) 38.9637 40.9618 nd has the largest (most negative) lattice energy? Which is a physical property of this MacBook Pro A) 7 00 8 9arrow_forward
- Formulas: V == E = hc/A Constants: h=6.626x10-34 c=2.998x108 Question 14 λ = h my A chemical bond between Ac and F would be O ionic because it is between a metal and nonmetal O metallic because it is between 2 metals O covalent because it is between two metals O ionic because it is between 2 nonmetals E= -2.18 x 10-18 J (1/²) and why?arrow_forwardQUESTION 1 Fill in the missing information: formulas for missing compounds and ions, systematic/modern names of compounds, and whether the compound is ionic (I), covalent (C) or both (B). { NOTE: i) Write subscript numbers in the formulas as if you are writing letters in a word (e.g., Mg3N2 should be written as Mg3N2). ii) Do not put parentheses around monatomic ions but put them around a polyatomic ion if there is more than one in the formula (e.g. Na2C03 should be written as Na2CO3 but Ca(NO3)2 as Ca(NO3)2. )} (Use the letters I, C, or B to indicate the type of bonding in the compound.) Compound Formula Name of compound lonic (I), Covalent (C) or Both (B)? lons 2- 1+ NH 4 & SO 4 4+ Sn & CI 1- 2+ Fe & CH 3 CO0 1- AI 3+ & p 3- AIParrow_forwardcan you explain and answer this for me please? and use the information for both Activites.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY