
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
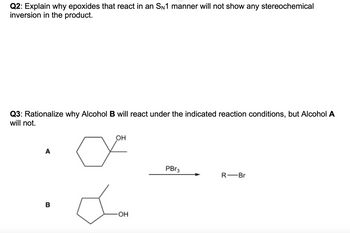
Transcribed Image Text:Q2: Explain why epoxides that react in an SN1 manner will not show any stereochemical
inversion in the product.
Q3: Rationalize why Alcohol B will react under the indicated reaction conditions, but Alcohol A
will not.
A
☑
OH
B
OH
PBr3
R-Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Select all compounds capable of keto-enol tautomerism. он H.C. он H.C. H.C. H.C. CH, CH, CH, H.C H.C a b darrow_forwardAnswer the following question ...arrow_forwardNonconjugated B,y-unsaturated ketones are in base-catalyzed equilibrium with their conjugated a,ß-unsaturated isomers. The mechanism of interconversion involves the formation of a resonance-stabilized intermediate with three resonance contributors. Draw the structures of these three resonance contributors. OH • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the symbol from the drop-down menu. + *** Sn [Farrow_forward
- Draw the organic products formed in each reaction.arrow_forwardComplete the following reactions. Where more than one product is possible, show only the one expected according to Markovnikovs rule. a. b.CH3CH2CH=CH2+Cl2 c. d.arrow_forward(a)Which reagent would help in this workup please NaCN CH3CO₂H aarrow_forward
- Is the product of this reaction more or less soluble than the initial molecule? Why? 1. RCO;H 2. H*, H,O Less water-soluble More water-soluble Water-solubility improved due to a negative charge on the product. Water-solubility improved due to a positive charge on the product. The product now has sufficient H-bonding functional groups to support water-solubility. An acetal has been formed which would decrease the water-solubility Another reasonarrow_forwardDraw a full arrow pushing mechanism the acid-catalyzed keto-enol tautomerization belowarrow_forwardHelp please explain how to do both please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
