
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
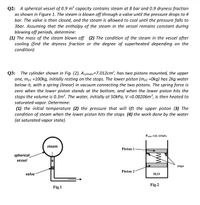
Transcribed Image Text:Q2: A spherical vessel of 0.9 m³ capacity contains steam at 8 bar and 0.9 dryness fraction
as shown in Figure 1. The steam is blown off through a valve until the pressure drops to 4
bar. The valve is then closed, and the steam is allowed to cool until the pressure falls to
3bar. Assuming that the enthalpy of the steam in the vessel remains constant during
blowing off periods, determine:
(1) The mass of the steam blown off (2) The condition of the steam in the vessel after
cooling (find the dryness fraction or the degree of superheated depending on the
condition)
The cylinder shown in Fig. (2), Acylinder=7.012cm², has two pistons mounted, the upper
Q3:
one, mp1 =100kg, initially resting on the stops. The lower piston (mp2 =Okg) has 2kg water
below it, with a spring (linear) in vacuum connecting the two pistons. The spring force is
zero when the lower piston stands at the bottom, and when the lower piston hits the
stops the volume is 0.3m³. The water, initially at 50kPa, V =0.00206m³, is then heated to
saturated vapor. Determine:
(1) the initial temperature (2) the pressure that will lift the upper piston (3) The
condition of steam when the lower piston hits the stops (4) the work done by the water
(at saturated vapor state).
Patm.=101.325kPa
steam
Piston 1
spherical
vessel
stops
Piston 2
valve
H2O
Fig.2
Fig.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A gas from a dry gas reservoir has a specific gravity of 0.735 at reservoir temperature of 200 degree Fahrenheit and reservoir pressure of 1800 psia. Determine the following: i. Gas compressibility factor for the dry gas.ii. Gas formation volume factor in res bbl/SCFarrow_forwardAssume that atmospheric temperature does not change with elevation but the atmospheric pressure decreases with increasing elevation. As a balloon filled with helium rises up into the sky, is it more likely to: A) expand B) contract C) stay the same size D) turn into a pumpkinarrow_forward04: A vertical, frictionless piston - cylinder device contains a gas at (500 KPa). The atmospheric pressure outside is (100 KPa), and the piston area is (30 cm²). Determine the mass of the piston. Assume standard gravitational acceleration? Ans.(122.32 kg)arrow_forward
- Re= 77419.2arrow_forwardThe atmospheric lapse rate on a particular day is constant in the lower part of the atmosphere. At ground level, the pressure is 1020 mBar and the temperature is 15°C. At a height z1 the pressure and temperature are 975 mBar and 11.5 °C. Determine the atmospheric temperature gradient, and the height z1.arrow_forward5. Solar ponds are small artificial lakes used to store solar energy. The rise of heated (and thus less dense) water to the surface is prevented by adding salt at the pond bottom. In a typical salt gradient solar pond, the density of water increases in the gradient zone, as shown. For H 4 m, po 1040 kg/m³, and a thickness of 0.8 m for the surface zone, calculate the gauge pressure at the bottom of the gradient zone. V S Sun = = H=4m = Surface zone Po 1040 kg/m³ Increasing salinity and density Gradient zone Storage zone - (2 Po=1040 kg/m P= Po(1+35+5²)arrow_forward
- When two objects at different temperatures are brought into contact with one another (such as a hot steel bar dropped into cold water), the cold object will O increase in temperature to the same temperature as the hot object before being placed in the water. O lose as much heat as the hot object loses the cold object will remain unchanged O gain as much heat as the hot object losesarrow_forwardTwo hundred kJ of work is transferred to the air by means of a paddle wheel inserted into an insulated volume . If the initial pressure and temperature are 200 kPa and 100 °C, respectively, determine the final temperature and pressure.arrow_forwardDetermine the efficiency for the cycle (standard air used as the working fluid) indicated in the sketch. Assume the pressures and temperatures are known quantities at each state. 1-2 constant volume process and 2-3 constant pressure process.arrow_forward
- May I ask if a block of copper falling into a tank filled with water is considered a closed or open steady system?arrow_forwardOn a day when wind is blowing over a lake (T(water) =20°C) at 5 m/s, which side of the air-water interface will be rate-limiting on the flux of Ethyl benzene (i.e. va or vw)?arrow_forward4. A mass of 1.7 kg of saturated liquid water is completely vaporized at a constant pressure of 101.325 kPa. Determine (a) the volume change and (b) the amount of energy transferred to the water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The