
Concept explainers
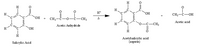
Q1. Using the structures of the reactants and products shown in the
a. Complete the following chemical equation by counting the number of atoms in each reactant and product and adding the subscripts where needed in the formula below.
(In essence, complete the formula of all the reactants and products.) (3)
C_H_O_ + C_H_O_ → C_H_O_ + C_H_O_
Salicylic acid + Acetic anhydride --> Acetylsalicylic acid (aspirin) + Acetic acid
b. Is this equation balanced or should it be balanced? If the equation is not balanced, balance it (1)
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Using the chemical formulas, calculate the molar mass of the reactant and product below.
Salicylic acid: _________ Acetylsalicylic acid: _________
Using the chemical formulas, calculate the molar mass of the reactant and product below.
Salicylic acid: _________ Acetylsalicylic acid: _________
- Can you please help me with question 4.arrow_forwardWhich of the following represents a combustion reaction? A. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) B. LiOH(aq) + HNO3(aq) → LiNO3(aq) + H2O(l) C. N2(g) + 3H2(g) → 2NH3(g) D. 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) E. 2Al(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g)arrow_forwardH2. Consider the following reaction: CH4 + 2O2 --> 2H2O + CO2 How many moles of water can be formed from 3.7 moles of CH4 and 3.0 moles of O2? Explain with detailsarrow_forward
- In the unbalanced chemical equation shown above for the combustion of ethanol, what least common multiple whole number coefficient should go in front of the hydrocarbon (C2H5OH) to balance the equation? See image belowarrow_forwardHere is a graph of the molarity of acetonitrile CH3CN in a reaction vessel during a certain chemical reaction. Use this graph to answer the questions in the table belowarrow_forwardEthanol (C,H O) is combusted in air according to the following reaction: C,H,O(1) + O,(g) –→ CO,(g) + H,0(I) How many grams of water would be produced by the complete combustion of 5.07 moles of ethanol in the presence of excess oxygen? + MacBook Pro 888 こニ。 esc & # $ @ 7 2 3 4 R Y Q W E ab F А lock C V option command ontrol cの つ つ エarrow_forward
- I need help understanding how to do this homework question. I've been out sick so I don't even know how to even begin to attempt this question.arrow_forwardUse the following information to answer this question. 2Al) + 3CuCl2(aq)→ 2AICI3(aq) + 3Cu(s) (word equation: aluminum plus three copper(Il) chloride produce two aluminum chloride and three copper) Which of the following best describes what is occurring during this reaction? Select one: O a. Aluminum replaces chlorine in the compound. O b. Aluminum replaces copper in the compound. O c. Copper replaces aluminum in the compound. O d. Chlorine replaces copper in the compound. Smoke Signal.pdf EFiles (1).zip Chap6 SF10 (1).pdfarrow_forwardIn the picture shown, the limited reactant is ____. The excesss reactant is ____. The product of the reaction is ___.arrow_forward
- Given the reaction: 2C,H, + 70, → 4C0, + 6H,0 What is the total number of moles of CO, produced by the complete combustion of 5.0 moles of C,H,? 2.0mol 1.0mol 5.0mol O 10.molarrow_forward< For the following reaction, 62.5 grams of silver nitrate are allowed to react with 27.9 grams of copper(II) chloride. silver nitrate (aq) + copper(II) chloride (s) → silver chloride (s) + copper(II) nitrate (aq) What is the maximum amount of silver chloride that can be formed? [Review Topics] [References] Use the References to access important values if needed for this question. What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? Submit Answer Retry Entire Group Show Hint 9 more group attempts remaining grams grams Previous Next Sve and Exit Save and Exitarrow_forwardWhen H,S(g) reacts with O2(g) to form H,0(g) and SO2(g), 124 kcal of energy are evolved for each mole of H,S(g) that reacts. Write a balanced equation for the reaction with an energy term in kcal as part of the equation.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





