
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
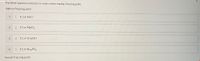
Transcribed Image Text:Put these aqueous solutions in order of decreasing freezing point.
highest freezing point
1
E 0.1 m NACI
| 0.1 m MgCl2
3
E 0.1 m CH3OH
| 0.1 m NazPO4
lowest freezing point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each row of the table below describes an aqueous solution at about 25 °C. Complete the table. That is, fill in any missing entries in the second and third columns. Be sure each entry you write includes the correct number of significant digits. solution H,0 pH Ox10 - 10 mol/L A 2.4 x 10 В ||mol/L 4.46 -7 5.7 х 10 mol/L Carrow_forwardA 10.27 g sample of a weak acid (HA) is dissolved in 509.0 g of water. The freezing point of the solution is -0.368 °C. The molar mass of the weak acid is 93.0 g/mol. Calculate the acid dissociation constant, K,, of the weak acid. K₁ = 8.17 X10-2 Incorrectarrow_forwardAssuming equal concentrations and complete dissociation, arrange these aqueous solutions from highest to lowest freezing points. - Fe2(CO3)3 - MgCl2 - KNO3 - Li3PO4arrow_forward
- Which of the following has the lowest freezing point? 0.150 m КCІ 0.150 m NaCI 0.150 m NaOH 0.150 m sugar 0.150 m CаCl2arrow_forwardEach row of the table below describes an aqueous solution at about 25°C . Complete the table. That is, fill in any missing entries in the second and third columns. Be sure each entry you write includes the correct number of significant digits. solution H3O+ pH A ×4.010−10mol/L B mol/L 4.14 C ×1.610−7mol/Larrow_forwardPlease type it or put check marksarrow_forward
- Which of the following aqueous solituions will have the highest freezing point 0.2 m AgNO3 0.3 m CaCl2 0.1 m LiCl 0.4 m CH4Oarrow_forwardThe density of an aqueous solution that is 13.2% by mass of XY ( molar mass 56.8 g/mol ) is 1.10 g/ mL. calculate the molarity of this solution.arrow_forwardA student determined the average molarity of their sample of acetic acid to be 0.611 M. Calculate the (w/w)% of the solution. Assume the density of this solution is that of pure water, dH2O = 1000. g/L. Enter a number without the '%' symbol.arrow_forward
- Predict the gas that is expected to have the highest molar concentration in aqueous solution at a fixed temperature and pressure? CO2 O Ne O H2 O N2arrow_forwardWhich of the following solutions will boil last under identical heating? (Do not forget that some things may dissociate, forming more ions) 0.020 M glucose O 0.020 M NaCl O 0.015 M CaCl₂ O 0.010 M HCI O 0,010 M Na₂Sarrow_forwardSuppose a 0.036M aqueous solution of phosphoric acid (H3PO4) is prepared. Calculate the equilibirum molarity of HPO4^2- Round your answer to 2 significant figures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY