
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
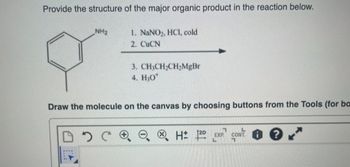
Transcribed Image Text:Provide the structure of the major organic product in the reaction below.
1. NaNO₂, HCl, cold
2. CuCN
NH₂
3. CH3CH₂CH₂MgBr
4. H₂O*
Draw the molecule on the canvas by choosing buttons from the Tools (for ba
DDCR
7
H: 120 EXP CONT?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. What is the mass percent of potassium chloride in a solution that is made by dissolving 15.0 g KCI in 100.0 g H,O? Show work. Parrow_forwardFor this reaction, 2 NH3 + CO2 --> H2NCONH2 + H2O how many moles of CO2 are required to produce 3.0 moles of H2NCONH2?arrow_forwardPlease help me out accurately its importantarrow_forward
- ds NURSE IN THE MAKING M OF THE MOST COMMON TIONS SEEN ON THE NCLEX ! TUL O SIMPLE REACTIONS Predicting the products of dissolution The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). -6 Note: "major" chemical species are those present in concentrations greater than 10 compound iron(III) bromide sodium cyanide sucrose Explanation TUL formula FeBr3 Na CN C12H22011 Check TUL Last 4 secil 7594 TXDP Priven 24 major species present when dissolved in water 0 Cera 0 0 pu Welona You got this !!! 5 Carlos de linn SalesAssets@vivint.com com X mol/L. 0₁ 5…arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help ») Thu Oct 14 7:04 PM B Homepage - CHM110-201 Gen X B CHEM101 HW #4 & 5 - CHM110 X 101 Chem101 app.101edu.co K Question 17 of 28 Submit How many moles of H,SO, are required to completely react with 7.20 | mol of Al 4 according to the balanced chemical reaction: 2 Al(s) + 3 H,SO,(aq) → Al,(SO,),(aq) + 3 H,(g) STARTING AMOUNT 98.08 342.14 2.02 7.20 26.98 10.8 3 21.6 3.60 1 2.40 6.022 х 1023 2 g Al,(SO,), g Al mol H2 g H,SO, g H2 mol H,SO, mol Al mol Al,(SO,), + 90 OCT 14 étv w 280arrow_forwardWhat is the effective mass yield for the reaction shown? The density of ethyl L-lactate is 1.03 g/mL. Round your answer to the nearest whole number.arrow_forward
- 6-63 Using each of the following balanced chemical equations, calculate the number of moles of the first listed reactant needed to produce 5.00 moles of CO2. a. C7H16 + 11O2 S 7CO2 + 8H2O Stoker, H. Stephen. General, Organic, and Biological Chemistry (p. 171-d).arrow_forwardPlease answer # 7 and if you are able to answer the second from # 6 (12gKClO3)arrow_forwardWhat reactant would transform the molecule from the top left to the top right. Given the same molecule, what would the given reactants transform it into below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY